 Videos
Videos
Christine Bain, Ph.D., shares how her role at KCAS Bio – Lyon, gives her the unique opportunity to work at the intersection of sponsor needs and the science behind it all.
 Videos
Videos
Magali Roche, Ph.D., shares her love for working with sponsors from all over the world to help improve health worldwide.
 Videos
Videos
Dawn Dufield, Ph.D. and Dominic Warrino, Ph.D. discuss their approach to ligand binding assays (LBAs) and hybrid mass spectrometry (Hybrid LC-MS/MS) in this 11-part video series. What Makes a Good CRO for Bioanalysis? Are there any specific technologies that are more useful to have together? What is a Hybrid…
 Posters & Papers
Posters & Papers
Discover here KCAS Bio’s work on monitoring the immunogenicity of a vaccine candidate by ELISpot.
 Posters & Papers
Posters & Papers
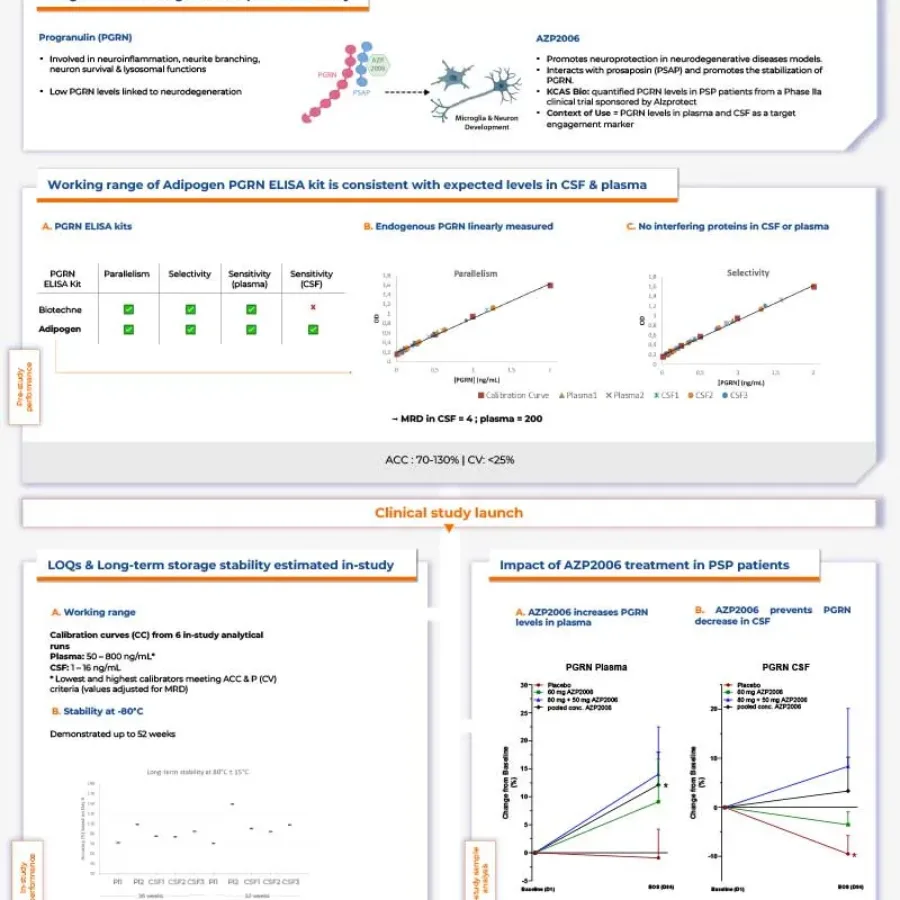
Please download this poster, “Performance of a method for quantifying progranulin as a target engagement biomarker for azp2006 in progressive supranuclear palsy.”
 Posters & Papers
Posters & Papers
Please download this poster, “Flow Cytometric Analysis of Urine Cell 18-Color Immunophenotyping Panel.”
 Podcasts
Podcasts
For episode #7 of “The Conversational Flow”, Brian and Adam invite David Ambrose to join them as they continue their conversation about Receptor Occupancy assays. There are so many unique considerations around an RO assay and with any flow cytometry assay, the precision of that assay is important. Our hosts…
 Blogs
Blogs
Over the past decade, a continued discussion point has been the idea around analyzing samples on Ligand Binding Assay (LBA) platforms in singlet (one well) versus the standard duplicate analysis (Single sample added to two different wells). In the recent M10 Bioanalytical Method Validation Guideline issued for guidance in June…
 Blogs
Blogs
At KCAS Bio, we continuously seek ways to improve our processes with sound, logical, scientific, and business-related methods. One of these areas of evaluation has been sample preparation. We have improved various segments of bioanalysis (drying for unstable analytes, liquid handling, and plate-based assays for increased efficiency). We can also…
 April 16
- April 17
April 16
- April 17
Dive into the world of biotechnology at the German Biotechnology Days conference, where experts and innovators converge to explore cutting-edge research and technologies. This dynamic event features keynote speakers, workshops, and thematic sessions covering genomics, synthetic biology, regenerative medicine, and more. Attendees can network with industry leaders, discover investment opportunities,…
 Blogs
Blogs
Pioneers of RNA Medicine: The Collaborative Journey of Katalin Karikó and Drew Weissman In the field of modern medicine, Katalin Karikó and Drew Weissman are recognized for their significant contributions to groundbreaking innovation, specifically within the areas of RNA biology and immunology. Their collaborative efforts paved the way for…
 Podcasts
Podcasts
CEO of KCAS Bio, John Bucksath, joins Dom and John for a special 75th episode of “The Weekly Bioanalysis” podcast, and he is absolutely delighted to use the special episode to talk about the recent rebrand of KCAS to KCAS Bio! Mr Bucksath and our hosts discuss the reason the…