Immunogenicity Testing Services
KCAS Bio has more than 12 years of experience in evaluating immunogenicity in both the non-GLP and GLP space. Using a multitiered approach, we can customize immunogenicity assays to fit your requirements through all phases of development.

Immunogenicity testing, a crucial step
Immunogenicity risk assessment is a crucial step in the development of therapeutic drug products. Immunogenicity assays are important because they allow us to understand the anti-drug antibody response to a patient's immune system which in turn helps us understand its safety and efficacy.

Immunogenicity experience
KCAS Bio has experience in assessing immunogenicity in a wide range of Biologics:
- Monoclonal Antibodies
- Antibody Fragments
- Antibody Drug Conjugates (ADCs)
- Fusion Proteins
- Gene Therapies
- Oligonucleotides
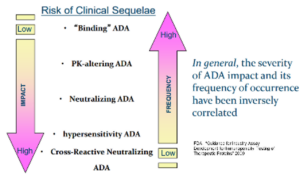
Immunogenicity assay development
KCAS Bio has a large (>60+ FTEs) ligand binding assay team that has 100s of years of combined experience with developing a wide range of immunogenicity assays from ADAs to cell based NAbs. Using a multitiered approach, KCAS Bio can customize your assay depending on your study requirements, through all phases of development.
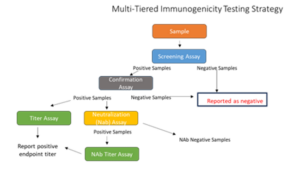
Tools to overcome immunogenicity challenges
Immunogenicity assays do not follow a one size fits all approach. We have the expertise to overcome challenges in immunogenicity assays caused by high background or insufficient drug tolerance starting at the experimental design, through method development/validation and into bioanalysis. Our team of scientists understand these challenges and have overcome them using a variety of techniques.
The most common assay formats are:
- Acid Dissociation
- Affinity Capture and Elution (ACE)
- Solid Phase Extraction and Acid Dissociation (SPEAD)
Acid
Dissociation
Acid dissociation is a widely used method for improving drug tolerance in an assay. When a weak acid is added to a sample it dissociates the drug: antibody complex. Once the sample is neutralized the ADA can bind to the biotinylated drug forming a bridge, this allows for the detection of the ADA. At KCAS Bio, we frequently add MgCl2 to help facilitate the breaking up of immune complexes.
Affinity Capture and Elution (ACE)
Affinity capture elution (ACE) is a method used in detecting ADA to increase the assay drug tolerance. The method uses acid disassociation of ADA-therapeutic interaction. This allows for preferential capture of ADA by an immobilized version of the dosed therapeutic. A second dissociation step is used to enable selective transfer of treated sample to an MSD bridging assay. Following the ACE procedure this assay utilizes biotinylated and ruthenium labeled drug to detect anti-drug antibodies.
Solid Phase Extraction & Acid Dissociation (SPEAD)
Solid Phase Extraction and Acid Dissociation (SPEAD) is a plate-based method for reducing drug interference in the ADA assay. This method uses weak acids to dissociate the drug: antibody complex. The sample is neutralized and binds a biotinylated drug which is then captured on the streptavidin-coated 96 well plate. After the plate is washed, a second acid treatment is used to elute the ADA bound to the plate. The sample containing the ADA is transferred, neutralized, followed by incubation with the labeled drug to form a bridge which is detected using ECL.
Statistical support for calculating cut points in immunogenicity testing
Interpreting results from Immunogenicity testing per guidelines can be challenging. We utilize the Red Thread™ module for Immunogenicity/Anti-drug Antibody (ADA) cut-point analysis. The Red Thread™ module uses the power of expert systems to simplify and automate the tedious, time-intensive and complicated process of analyzing cut-points for ADA studies.
Tell us how we can help with your immunogenicity project
We've earned our reputation for delivering reliable, error-free data. We understand the importance of speed, flexibility, and consistency and only make promises we can keep.
Resources & insights
 Blogs
Blogs
Initially developed to assess the frequency of circulating antigen-specific Antibody-Secreting Cells (ASC), ELISpot has become a vital tool for quantifying antigen-reactive T-cells by measuring secreted immune mediators such as cytokines or key molecules involved in cell-mediated cytotoxicity. Compared to other assays for monitoring cell-mediated immunity (CMI), such as…
 Blogs
Blogs
What Are Neutralizing Antibody (NAb) Assays? Neutralizing antibodies (NAbs) are a subset of anti-drug antibodies (ADAs) that play an important role in evaluating the immunogenicity, safety, and efficacy of a drug product. While most drugs carry a low risk of eliciting NAbs and do not require a validated NAb…
 Blogs
Blogs
Immunogenicity, why do we keep talking about it? Immunogenicity can, in the simplest of terms, be described as a subject’s ability to generate antibodies specific to the dosed protein therapeutic. While stating it this way understates the complexity of the immune system and the methods needed to detect and characterize…

Agile, responsive, and easy to work with
We prepare and adapt our services based on a deep understanding of your drug development ambitions and wider business objectives.