 Blogs
Blogs
In the process of drug discovery, an IC₅₀ assay serves as a pivotal component in the development of cutting-edge therapeutics. An effective assay can quantify the potency of compounds, enable side-by-side comparisons with competing compounds, and track how a compound performs over time. Quantifying compound inhibition also assists with pre-clinical…
 September 30
- October 2
September 30
- October 2
Join KCAS Bio at the 2025 Festival of Biologics, taking place from September 30 to October 2 in Basel, Switzerland. This festival, hosted by Terrapinn, brings together global stakeholders across pharma, biotech, academia, regulation, and investment to explore the discovery, development, manufacturing and commercialization of biologic therapies. During presentations, panels,…
 September 26
- September 30
September 26
- September 30
KCAS Bio will be attending the International Clinical Cytometry Meeting (ICCS) 2025 on September 26-30 in Philadelphia, PA. This annual meeting brings together leaders and scientists to advance the understanding and application of technologies and methodologies in patient sample analysis. Through presentations and collaborative discussions, attendees will explore the latest…
 September 14
- September 17
September 14
- September 17
KCAS Bio will be attending the EUROTOX Congress 2025, scheduled for September 14-17 in Athens, Greece. Organized by the Federation of European Toxicologists and European Societies of Toxicology, this event will bring together leading experts and emerging scientists to explore practical approaches to sustainable health and well-being. The gathering will…
 September 8
- September 11
September 8
- September 11
Join KCAS Bio at the 26th International Reid Bioanalytical Forum, taking place from September 8-11, 2025, in Cambourne, UK. This event, hosted by Neil Spooner and Tim Sangster, will bring together professionals and academics to exchange perspectives on current practices and challenges in bioanalysis. Featuring presentations and discussions from organizations…
 Podcasts
Podcasts
In this episode of “The Weekly Bioanalysis” podcast, our hosts, Dominic Warrino and John Perkins, sit down with two key leaders shaping the company’s future — Maria Nelson, Chief Financial Officer, and Julie Deane, Chief People Officer — to discuss KCAS Bio’s strategy for balancing rapid growth with an exceptional…
 Podcasts
Podcasts
In episode 21 of “The Conversational Flow” podcast, our hosts, Adam and Brian, explore the transformative power of gene therapy, starting things off by highlighting a compelling case involving CRISPR with milestones that once seemed impossible. They dive into the science behind CRISPR’s precise gene-editing capabilities and its potential for…
 Blogs
Blogs
PCR-based assays are increasingly utilized for bioanalysis to support the development of a wide variety of therapeutics. While the largest driver for this growth has been the expanding pipeline of cell, gene, and RNA therapies, PCR-based assays are also seeing increased use in biomarker detection across all therapeutic modalities. These…
 Blogs
Blogs
Remember that childhood excitement the night before Christmas? Too excited to sleep, knowing something you wished for was finally within reach? That’s exactly the spirit fueling our teams in Lyon as we approach the end of June. We’re eagerly awaiting the arrival of a powerful new platform at our…
 Posters & Papers
Posters & Papers
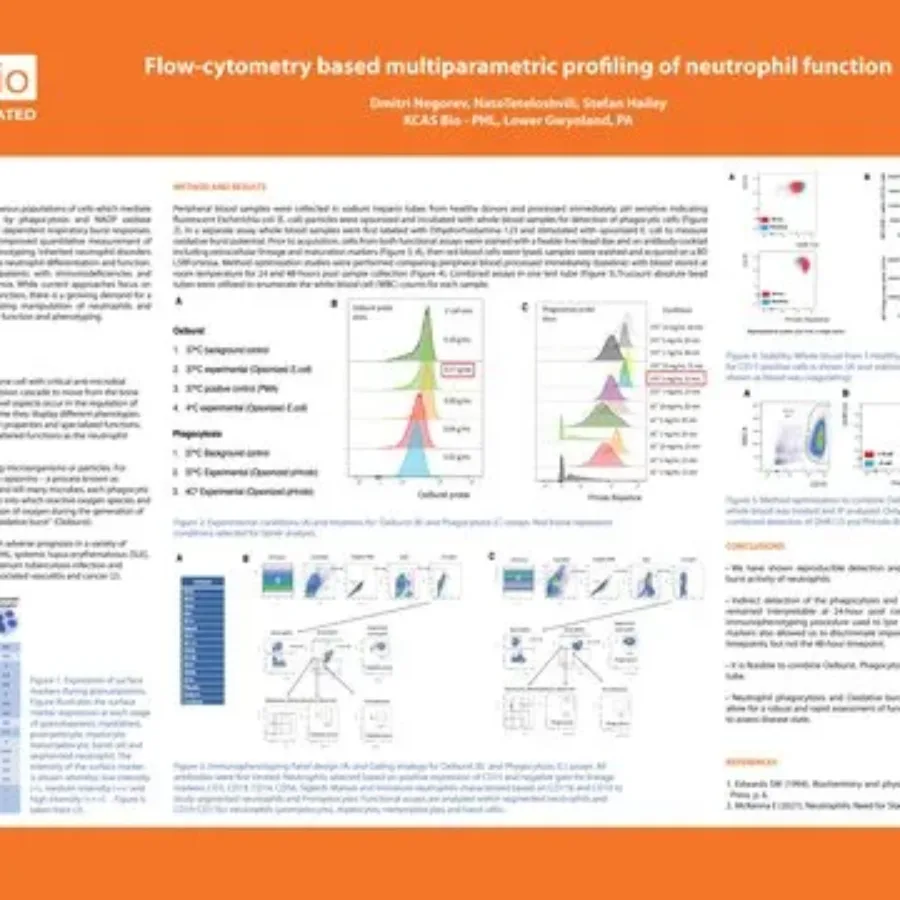
Discover in this poster presented by Dmitri Negorev at CYTO 2025 on the “Flow-cytometry based multiparametric profiling of neutrophil function”. Flow-cytometry Based Multiparametric Profiling of Neutrophil FunctionDownload If you have any questions about these services or any others offered by KCAS Bio,…
 Blogs
Blogs
Hybrid LC-MS/MS is a technique that combines an affinity capture step with LC-MS/MS detection. It typically requires only one antibody, in contrast to conventional ligand-binding assays (LBAs), which usually need two. This approach leverages the combined selectivity of affinity extraction and the analytical power of tandem mass…
 Blogs
Blogs
Biomarkers (BMKs) have become fundamental tools in drug development, accelerating and optimizing targeted therapeutic innovation. As a bioanalytical CRO with over 15 years of experience in biomarker analysis, we’ve seen firsthand how the strategic integration of biomarkers can accelerate drug programs, help meet evolving regulatory standards,…