 June 25
- June 26
June 25
- June 26
KCAS Bio is happy to announce our participation in the 13th edition of the Antibody Industrial Symposium (AIS), taking place in Tours, France. As a premier event in the antibody and therapeutic development sector, AIS offers a unique platform for scientific exchange, collaboration, and innovation. KCAS Bio’s Expertise…
 Podcasts
Podcasts
In Episode 89 of The Weekly Bioanalysis, hosts Dominic Warrino and John Perkins engage in a compelling conversation with special guest, Jeff Goddard, Senior Vice President of Corporate Development at KCAS Bio. The three delve into KCAS Bio’s strategic growth, including the acquisition of France-based Active Biomarkers, which expanded the company’s transatlantic…
 Blogs
Blogs
From initial receipt to final disposition, sample integrity, traceability, and compliance are the foundation of our bioanalytical operations. We’re taking you behind the scenes to follow the journey of a sample through our facility. Here’s a detailed look at each stage in the lifecycle of a study sample at KCAS…
 Podcasts
Podcasts
In this milestone 20th episode of “The Conversational Flow” podcast, hosts Adam and Brian dive into impactful developments across oncology, regulatory science, and bioanalytical innovation. The episode begins with a highlight of a recent major Keytruda study showing the first significant improvement in head and neck squamous cell carcinoma outcomes…
 Blogs
Blogs
KCAS Bio participated in the AAPS National Biotechnology Conference (NBC), held from May 4 to May 7, 2025, in Boston. It was a productive event that allowed us to connect with sponsors, exchange ideas with peers, and present our latest work in bioanalytical science. Our experts…
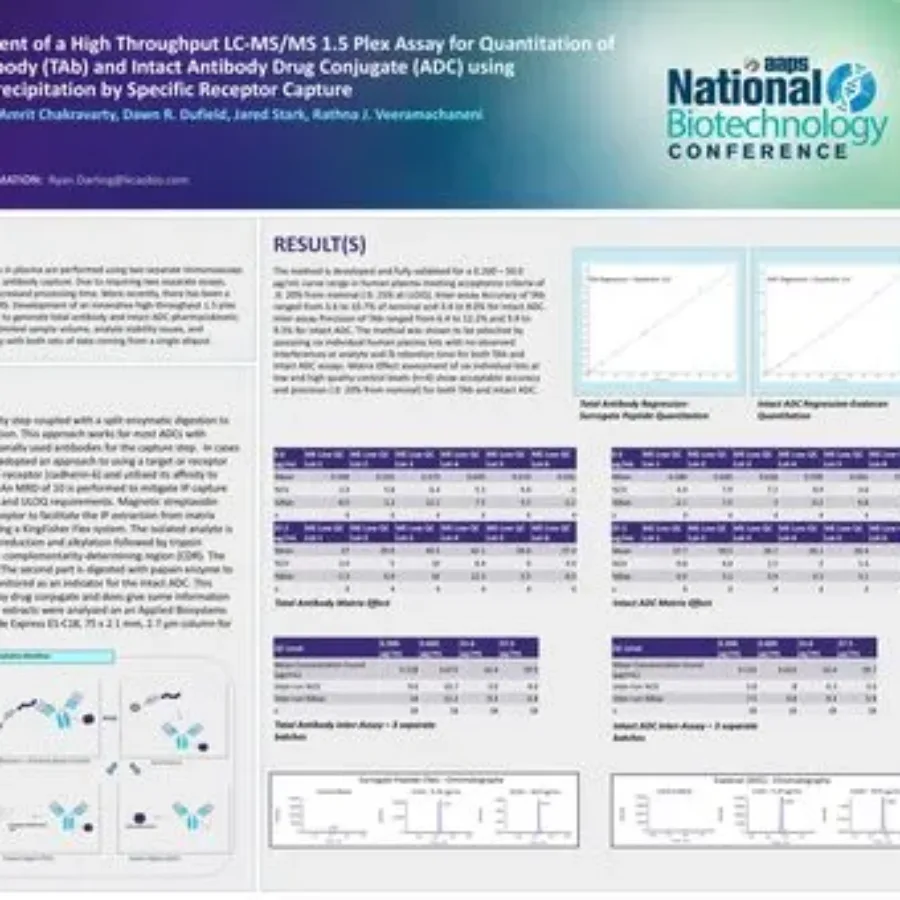 Posters & Papers
Posters & Papers
Discover in this poster presented by Ryan Darling at the AAPS National Biotechnology Conference 2025 on the use of a hybrid LC-MS/MS approach for the quantitation of total antibody TAb and intact Antibody Drug Conjugate (ADC). HybridLC-MSMSAssayforADCQuantitation_KCASBioDownload If you have any…
 Blogs
Blogs
In drug development, the journey from target identification to clinical candidate selection involves a series of critical steps, including compound screening, lead optimization, and pre-clinical testing. Each stage helps narrow the pool of potential therapeutics, with the goal of identifying the most promising candidates for clinical evaluation. Given the time,…
 Blogs
Blogs
At KCAS Bio, we offer expert flow cytometry services designed to support the evolving bioanalytical needs of immunology and biomarker projects for drug development. Whether you’re working in preclinical or clinical settings, our validated off-the-shelf panels save time, reduce risk, and deliver high-quality, reproducible data you can trust. Extensive Portfolio…
 Podcasts
Podcasts
In Episode 88 of The Weekly Bioanalysis, hosts Dominic Warrino, Ph.D. and John Perkins, Ph.D present a special WRIB 2025 recap featuring several KCAS Bio scientists who share firsthand insights from their presentations at the major bioanalytical conference. David Ambrose highlights KCAS Bio’s global harmonization of spectral flow cytometry instruments across…
 Blogs
Blogs
KCAS Bio offers a wide range of biomarker services, from cell-based to soluble biomarker analysis, including ligand binding assays (LBA), across a variety of matrix types. Soluble biomarker analysis can be achieved on multiple platforms depending on factors such as sample type, required sensitivity, and whether multiplexing is…
 Podcasts
Podcasts
In the 19th episode of “The Conversational Flow”, our hosts, Brian and Adam, dive into the utility and innovation behind backbone panels in spectral flow cytometry, spotlighting a recent paper from BMS that presents a robust, flexible assay designed for immune monitoring using the Aurora platform. They discuss how backbone…
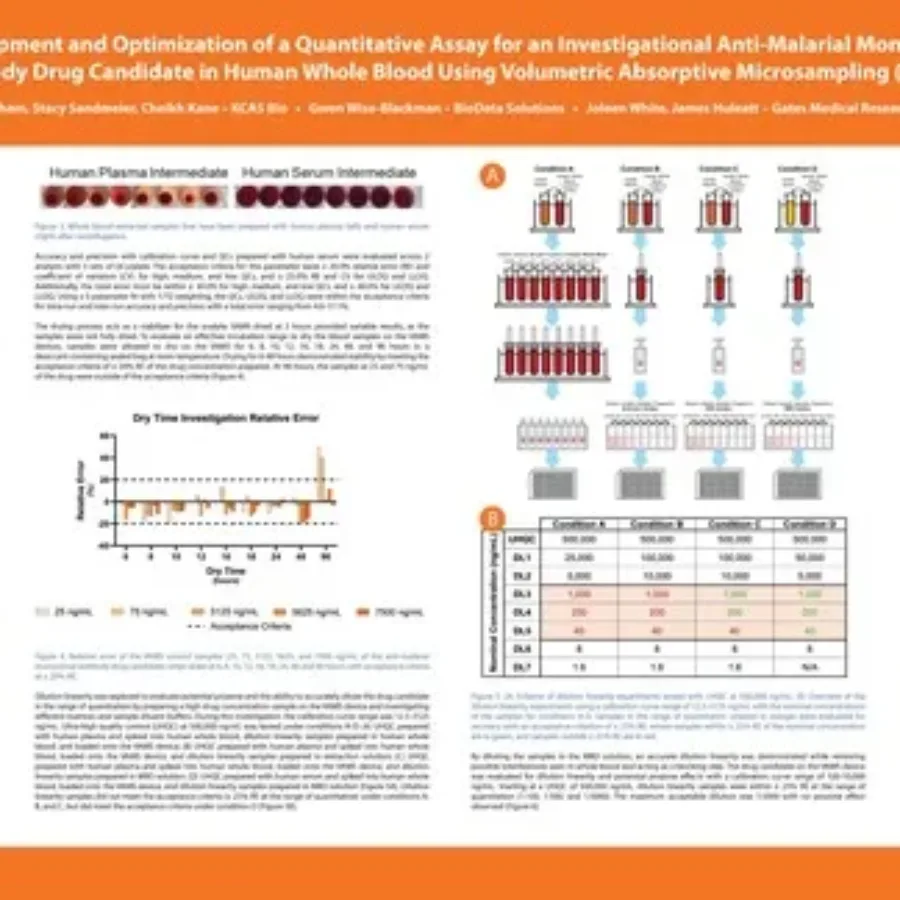 Posters & Papers
Posters & Papers
Discover in this poster presented by Jessica Phan at WRIB 2025 on the “Development and Optimization of a Quantitative Assay for an Investigational Anti-Malarial Monoclonal Antibody Drug Candidate in Human Whole Blood Using Volumetric Absorptive Microsampling (VAMS)”. Quantitative Assay for an Investigational Anti-Malarial Monoclonal Antibody Drug Candidate |…