You searched for "hybrid"
 Blogs
Blogs
Hybrid LC-MS/MS is a technique that combines an affinity capture step with LC-MS/MS detection. It typically requires only one antibody, in contrast to conventional ligand-binding assays (LBAs), which usually need two. This approach leverages the combined selectivity of affinity extraction and the analytical power of tandem mass…
 Blogs
Blogs
ADCs represent a promising class of targeted therapies, and understanding the intricacies of their analysis is crucial for their successful development. In this blog, we address common questions to guide you through the various considerations involved in ADC research and testing. We will cover key topics related to the…
 Blogs
Blogs
Anyone who has been following KCAS for any amount of time has likely heard us discuss the advantages of Hybrid LC-MS/MS for the bioanalysis of large molecules. As pioneers in the technology for many years, KCAS has become a strong proponent of working with our customers to demonstrate the quality…
 Blogs
Blogs
Due to the relative maturity of the technology, most people’s instinct when thinking about bioanalysis of large molecules is towards ligand binding assays. However, we urge people to be open to a more flexible approach. Using hybrid LC-MS/MS can be an equally useful tool for quantitation of biological therapeutics and…
 November 15
- November 17
November 15
- November 17
For years, Dawn Dufield, PhD and I have been doing our best to get the word out (via as many venues as possible) about KCAS’s unique expertise with hybrid LC-MS/MS. This year’s upcoming EBF meeting in Barcelona is just such an opportunity, and one we are very much looking…
 Webinars
Webinars
As the pharmaceutical industry continues to evolve, so does the technology behind drug development. One of the most groundbreaking advancements in bioanalysis is Hybrid LC-MS/MS technology, which is rapidly becoming the new standard for analyzing Antibody Drug Conjugates (ADCs). KCAS Bio is proud to be at the forefront of this…
 Webinars
Webinars
For researchers and professionals working in the biopharmaceutical industry, an upcoming Bioanalysis Zone Panel Discussion will offer a unique opportunity to stay informed about the latest advancements in quantitative analysis techniques. Research projects can only succeed when selecting the appropriate biopharmaceutical quantification technique. Staying up-to-date on…
 Webinars
Webinars
Originally produced by Xtalks on Friday, April 22, 2022 | 12pm EDT (NA) / 5pm BST (UK) / 6pm CEST (EU-Central) 60 min Webinar Description: Due to the relative maturity of the technology, most people’s instinct when thinking about bioanalysis of large molecules is towards ligand binding assays.
 Blogs
Blogs
There has been an increasing focus on large molecule therapeutics and pharmaceutical companies have increasingly aligned their development pipelines in that direction. This has resulted in more biological therapeutics coming to market. Last year,…
 Blogs
Blogs
Antibody-drug conjugates (ADCs) have been conventionally developed for a wide range of oncological applications since the first ADC approval in 2000 by the FDA. Typically, an ADC consists of three main components: antibody, linker conjugate, and therapeutic drug (payload). The mechanism of an ADC involves the antibody binding to a specific…
 Blogs
Blogs
The age of therapeutic conjugation is upon us! Bioanalysis for support of next-generation Antibody Drug Conjugates (ADCs) and Antibody siRNA Conjugates (ARCs) have exploded recently due to the efficacy and safety that these therapies offer for immuno-oncology, rare diseases, vaccines, and potentially many other diseases. Recently, we have seen the…
 Blogs
Blogs
In the complex and dynamic bioanalytical landscape of antibody-drug conjugate (ADC) services, where often time is critical and every research dollar counts, hybrid technology is quickly emerging as a game-changer. Hybrid offers a spectrum of advantages that redefine traditional approaches to bioanalytical ADC drug development. At the forefront of these…
 June 20
- June 21
June 20
- June 21
The age of therapeutic conjugation is upon us! Bioanalysis for support of next generation Antibody Drug Conjugates (ADCs) and Antibody siRNA Conjugates (ARCs) have exploded recently due to the efficacy and safety that these therapies offer for immuno-oncology, rare diseases, vaccines and potentially many other diseases. Recently, we have seen…
 Blogs
Blogs
If you are part of the Biotech/Pharma community, you may be familiar with the (J.P. Morgan) JPM Healthcare Conference that takes place annually in January and typically in San Francisco. This was the 42nd Annual JPM Healthcare Conference and it is the world’s largest healthcare symposium. This year the mood…
 Videos
Videos
Dawn Dufield, Ph.D. and Dominic Warrino, Ph.D. discuss their approach to ligand binding assays (LBAs) and hybrid mass spectrometry (Hybrid LC-MS/MS) in this 11-part video series. What Makes a Good CRO for Bioanalysis? Are there any specific technologies that are more useful to have together? What is a Hybrid…
 Videos
Videos
Is Hybrid LC-MS/MS emerging as an Equal Partner? Click here to view the entire series.
 Videos
Videos
What is a Hybrid LC-MS/MS? Click here to view the entire series.
 Podcasts
Podcasts
Special guests Dawn Duffield, PhD of KCAS and Barry Jones, PhD of Crinetics join John and Dom in the 66th episode of “The Weekly Bioanalysis” to discuss the ways Hybrid LC-MS/MS has advanced in recent years and how the technology has gone from “new” and somewhat obscure to…
 Blogs
Blogs
In biomarker-driven drug development, selecting the right assay format is critical for generating reliable data that informs scientific and clinical decisions. Whether quantifying proteins, immune cell subsets, or complex molecular signatures, the choice between off-the-shelf and customized assays has direct implications for timelines, costs, and how well the assay performance…
 Blogs
Blogs
Drawing on the insights of our leadership team, we’ve compiled a global perspective on the state of the bioanalytical industry in 2024. Through thoughtful discussions with our CEO, John Bucksath, and key team members Amy Mize, Mouhssin Oufir, and Brian Wile, KCAS Bio delivers a roadmap…
 Blogs
Blogs
When it comes to developing new therapies for inflammatory bowel diseases (IBD), having robust and reliable bioanalytical support is crucial. From the early stages of drug discovery to Phase III clinical trials, our comprehensive bioanalytical solutions are designed to enhance and accelerate your drug development journey in a GCP…
 May 31
- June 5
May 31
- June 5
KCAS Bio is thrilled to announce our participation in the 73rd Conference on Mass Spectrometry and Allied Topics (ASMS), taking place in Baltimore, Maryland, from May 31 – June 5, 2025. This prestigious event is a cornerstone in the field of mass spectrometry, bringing together experts, innovators, and visionaries to…
 Blogs
Blogs
Drawing on the insights of our leadership team, we’ve compiled a global perspective on the state of the bioanalytical industry in 2024. Through thoughtful discussions with our CEO, John Bucksath, and key team members Amy Mize, Mouhssin Oufir, and Brian Wile, KCAS Bio delivers a roadmap…
 Blogs
Blogs
Oligonucleotide therapeutics have recently gained popularity as noted by recent increases in regulatory approvals of oligonucleotides as drugs, novel liquid nanoparticle delivery approaches, and on-target specificity. In the development of bioanalytical assays, there are many challenging aspects to consider when quantitating oligonucleotides, such as non-specific binding, stability, efficient ionization, sequence…
 June 2
- June 6
June 2
- June 6
KCAS Bio is excited to announce our participation in the 72nd ASMS Conference on Mass Spectrometry and Allied Topics, taking place in Anaheim, California, from June 2-6, 2024. This prestigious event brings together experts and enthusiasts from around the world to discuss the latest advancements and innovations in mass spectrometry.
 Blogs
Blogs
Ligand binding assays (LBAs) have been our core activity for decades. LBAs are commonly used to measure interactions between two proteins, a ligand and its receptor, a monoclonal antibody (mAb) and its target, or biologics and Anti-Drug Antibodies (ADA). Throughout the development of New…
 Blogs
Blogs
To read part one of this 2-part series, click here. With increasing focus on large molecule therapeutics and interest in large molecule biomarkers it’s essential to have LC/MS available as a complimentary technology to ligand…
 Blogs
Blogs
Biomarker is a broad term and can mean so many different things depending upon the scientific discipline. For Bioanalytical biomarker analysis – and specifically for CROs equipped with 9 different platforms to measure biomarkers and a cell culture suite with up to 19 color flow cytometry all under one roof…
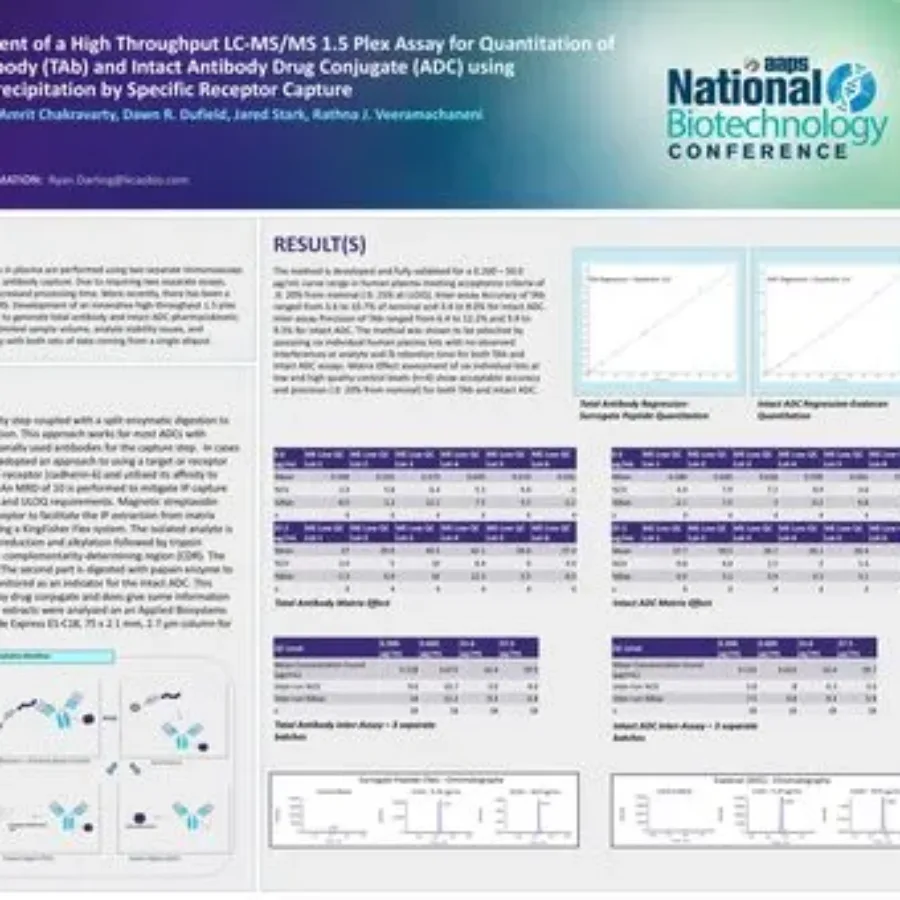 Posters & Papers
Posters & Papers
Discover in this poster presented by Ryan Darling at the AAPS National Biotechnology Conference 2025 on the use of a hybrid LC-MS/MS approach for the quantitation of total antibody TAb and intact Antibody Drug Conjugate (ADC). HybridLC-MSMSAssayforADCQuantitation_KCASBioDownload If you have any…
 Videos
Videos
Dom & Dawn’s Opinion on the CRO Industry? Click here to view the entire series.
 Videos
Videos
Do You Feel that Many Bioanalytical CROs are Quick to Cover All of These Technologies? Click here to view the entire series.
 Videos
Videos
What are Some Scenarios Where More Than One Approach is Needed? Click here to view the entire series.
 Videos
Videos
Do You Have Examples of When You Started with One Testing Platform and Switched to Another? Click here to view the entire series.
 Videos
Videos
How do You Know Which Approach is Best? Click here to view the entire series.
 Videos
Videos
Are the Sensitivities Similar? Click here to view the entire series.
 Videos
Videos
What are the Differences Between Time and Costs for Development? Click here to view the entire series.
 Videos
Videos
Are there any specific technologies that are more useful to have together? Click here to view the entire series.
 Videos
Videos
What Makes a Good CRO for Bioanalysis? Click here to view the entire series.
 Blogs
Blogs
Biomarker are key drivers in the drug development process. Frequently, developing a panel of biomarkers to test a particular mechanism or hypothesis is critical to the success of a drug. At KCAS, we develop assays for custom panels or individual biomarkers based on client needs and can also assist with…
 Blogs
Blogs
KCAS Bio participated in the AAPS National Biotechnology Conference (NBC), held from May 4 to May 7, 2025, in Boston. It was a productive event that allowed us to connect with sponsors, exchange ideas with peers, and present our latest work in bioanalytical science. Our experts…
 Webinars
Webinars
As the field of biotherapeutics rapidly evolves, the development of advanced conjugated therapies such as Antibody-Drug Conjugates (ADCs), Antibody-RNA Conjugates (ARCs), siRNA/oligos, and antibody-peptide conjugates has gained significant momentum. These next-generation therapeutics offer promising efficacy and safety profiles for treating various conditions, including cancer, rare diseases, and in vaccine development.
 Blogs
Blogs
The bioanalysis world has exploded with the need for molecular assays (qPCR, dPCR, NGS, Hybridization technologies) due to the demand for both biodistribution/PK and PD/BM analysis of various drug modalities. Many of these molecular assays have been around for decades and are now routine methods. CLIA and reference labs have…
 Blogs
Blogs
KCAS Bio is excited to expand our global reach by attending key conferences across Europe in 2025. These events bring together top experts in biotechnology, pharmaceuticals, and clinical research, providing a unique opportunity to discuss cutting-edge advancements, regulatory insights, and innovative solutions in bioanalytical and biomarker services. As we continue…
 Blogs
Blogs
KCAS Bio is thrilled to announce our participation in several key scientific and bioanalytical events across the United States in the coming months. These conferences provide a unique platform to showcase our cutting-edge capabilities, engage with industry leaders, and explore innovative approaches to bioanalysis. From gene therapy to toxicology and…
 Podcasts
Podcasts
After such a great response to our recent episode on the topic, our hosts, Dom and John, revisit the topic of GLP-1 Analogue development and the role bioanalysis plays in this relatively new and popular topic. In the words of John, “Everyone else is talking about this; why shouldn’t we?”…
 November 20
- November 22
November 20
- November 22
KCAS Bio is happy to announce our participation in the 17th EBF Open Symposium, taking place in Barcelona, Spain, from November 20-22, 2024. This prestigious event is a cornerstone for professionals in bioanalysis, offering a platform to share cutting-edge research, innovative methodologies, and industry best practices. We are thrilled to…
 July 22
- July 25
July 22
- July 25
KCAS Bio is excited to announce our participation in the AAPS Summer Scientific Forum, held in Kansas City, Missouri. As a leading event in pharmaceutical science, this forum provides a unique opportunity for professionals to share knowledge, explore innovative research, and collaborate on the latest advancements in the industry. We…
 June 20
- June 21
June 20
- June 21
KCAS Bio is happy to announce our participation in the 12th edition of the Antibody Industrial Symposium (AIS), taking place in Montpellier, France. As a premier event in the antibody and therapeutic development sector, AIS offers a unique platform for scientific exchange, collaboration, and innovation. We are excited to be…
 Blogs
Blogs
Dawn Dufield, PhD is the Scientific Officer for Mass Spectrometry and has been with KCAS Bio since 2018. Before joining KCAS Bio, she worked in the quantitative large and small molecule LC-MS/MS field for Pfizer for over 20 years. Dawn has been a pioneer in the Hybrid LCMS field and…
 May 15
- May 16
May 15
- May 16
Knowledge for Growth (KfG) is Europe’s premier life sciences conference, held annually in Antwerp, Belgium. This year’s edition celebrates the industry’s advancements with a hybrid format, catering to both in-person and virtual attendees. The program features captivating talks, scientific sessions on cutting-edge topics like gene editing and personalized medicine, and…
 Webinars
Webinars
Produced by AAPS Organized by Sciex Recorded on December 6, 2023 eChalkTalk available here. Webinar Description: In this webinar, scientists from KCAS Bio, along with specialists from SCIEX Biopharma & Pharma Solutions discuss • Developing robust assays for PD biomarkers and their challenges…
 November 15
- November 17
November 15
- November 17
Along with a series of studies we plan to present related to our experiences with hybrid LC-MS/MS technology, KCAS will also be presenting on a study where we analyzed a compound that involved looking at urea in plasma and lung fluid (urea, of course, being a biomarker). Urea is endogenous…
 Blogs
Blogs
With recent guidance released from the FDA, there are changes for PKs (Pharmacokinetics) and ADCs (Antibody Drug Conjugates) that must be clearly understood before making decisions for your drug product testing. ADCs combine the target specificity of monoclonal antibodies with the…
 Blogs
Blogs
With the extensive advances in technologies like CRISPR and CAR-T, cell and gene therapy has grown to become a viable way for treating Cancer as well as other diseases. Our team has over 100+ years of collective expertise in molecular services using qPCR and ddPCR for support of…
 Blogs
Blogs
Pharmacodynamics (PD) is defined as the study of the biochemical and physiological effects of drugs and the mechanisms of their actions. Where pharmacokinetics looks at how the organism processes the drug, pharmacodynamics studies how…
 Blogs
Blogs
KCAS is a solution-based company. We are focused on the delivery of high quality scientific and defendable data, able to meet or exceed client’s timelines and expectations. One of the main reasons we are able to deliver on even the most challenging projects and programs, is the technical breadth and…
 Blogs
Blogs
The 505(b)(2) drug development path, in contrast to traditional development of a new, never been approved drug (described under 505(b)(1) of the Federal Food, Drug, and Cosmetic Act) or ANDA (described under Section 505(j) of the Act) for a generic drug that is no longer under patent…