 October 17
- October 21
October 17
- October 21
KCAS Bio will attend the ESMO Congress 2025, taking place in Berlin, Germany on October 17-21. ESMO Congress brings together a diverse community in the oncology field to exchange knowledge, spotlight innovation, and shape the future of cancer care on a global scale. The meeting is an opportunity to connect…
 Blogs
Blogs
In the ever-evolving landscape of drug development, biomarkers have emerged as critical tools for the mechanism of action, early proof of mechanism, safety, predictive, efficacy, and monitoring treatment response. As the field advances, two major classes of biomarkers have come to the forefront: soluble biomarkers and cellular biomarkers. While…
 Blogs
Blogs
Remember that childhood excitement the night before Christmas? Too excited to sleep, knowing something you wished for was finally within reach? That’s exactly the spirit fueling our teams in Lyon as we approach the end of June. We’re eagerly awaiting the arrival of a powerful new platform at our…
 Blogs
Blogs
Biomarkers (BMKs) have become fundamental tools in drug development, accelerating and optimizing targeted therapeutic innovation. As a bioanalytical CRO with over 15 years of experience in biomarker analysis, we’ve seen firsthand how the strategic integration of biomarkers can accelerate drug programs, help meet evolving regulatory standards,…
 June 25
- June 26
June 25
- June 26
KCAS Bio is happy to announce our participation in the 13th edition of the Antibody Industrial Symposium (AIS), taking place in Tours, France. As a premier event in the antibody and therapeutic development sector, AIS offers a unique platform for scientific exchange, collaboration, and innovation. KCAS Bio’s Expertise…
 Podcasts
Podcasts
In Episode 88 of The Weekly Bioanalysis, hosts Dominic Warrino, Ph.D. and John Perkins, Ph.D present a special WRIB 2025 recap featuring several KCAS Bio scientists who share firsthand insights from their presentations at the major bioanalytical conference. David Ambrose highlights KCAS Bio’s global harmonization of spectral flow cytometry instruments across…
 Blogs
Blogs
KCAS Bio offers a wide range of biomarker services, from cell-based to soluble biomarker analysis, including ligand binding assays (LBA), across a variety of matrix types. Soluble biomarker analysis can be achieved on multiple platforms depending on factors such as sample type, required sensitivity, and whether multiplexing is…
 Blogs
Blogs
Quantitative flow cytometry (QFCM) is a specialized technique that enables precise measurement of the absolute number of specific molecules (e.g., receptors, antigens, or intracellular targets) on individual cells or particles. Understanding flow cytometry is essential, as standard methods typically provide qualitative data, where the relative fluorescence intensity is used to…
 Blogs
Blogs
We were happy to participate in the AD/PD™ 2025 International Conference on Alzheimer’s and Parkinson’s Diseases and related neurological disorders, held from April 1 to 5, 2025, in Vienna, Austria. The conference was a valuable opportunity to connect with experts, share our experience, and gain insights…
 Blogs
Blogs
Initially developed to assess the frequency of circulating antigen-specific Antibody-Secreting Cells (ASC), ELISpot has become a vital tool for quantifying antigen-reactive T-cells by measuring secreted immune mediators such as cytokines or key molecules involved in cell-mediated cytotoxicity. Compared to other assays for monitoring cell-mediated immunity (CMI), such as…
 Posters & Papers
Posters & Papers
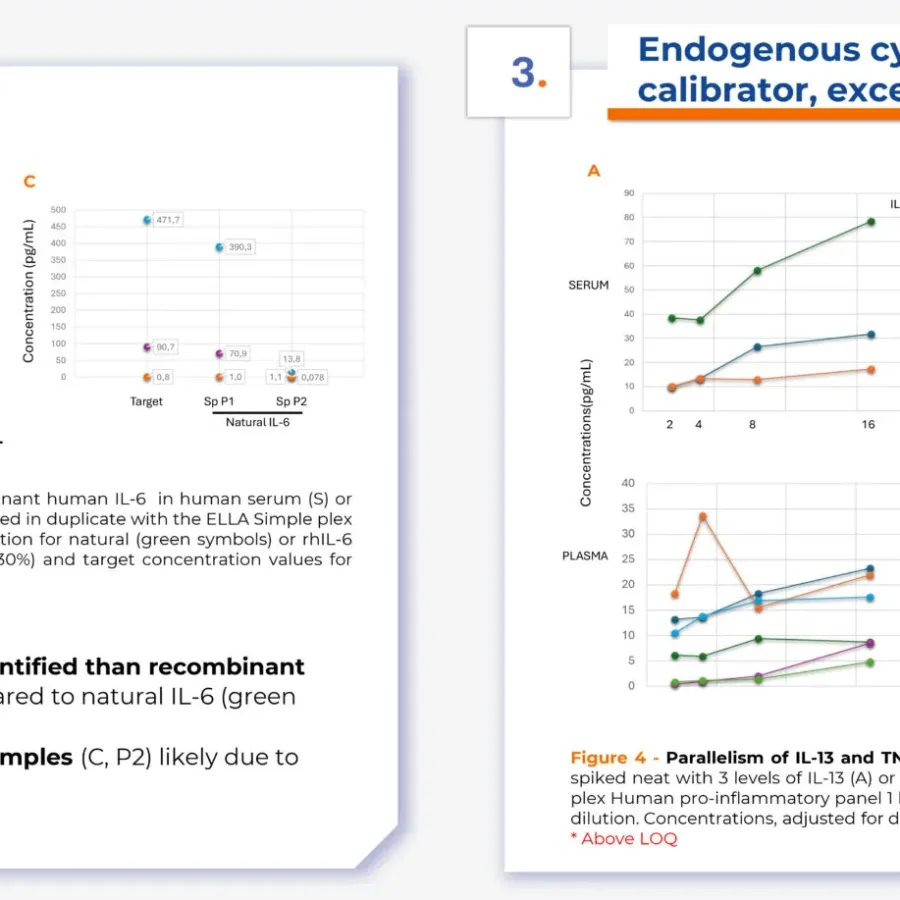
Discover innovative insights into bioanalytical method development with our poster, presented by Christine Bain, Ph.D., at ECCO 2025. This work explores the creation and application of reference samples containing endogenous immune mediators—such as cytokines and chemokines—to effectively evaluate the performance of bioanalytical methods. Download the poster now to learn more…
 Podcasts
Podcasts
In episode 87 of The Weekly Bioanalysis podcast, John Perkins and Dawn Dufield preview the upcoming WRIB conference, where KCAS Bio and Sciex will both have strong representation through scientific presentations, panels, and business development efforts. Special guest, Rahul Baghla of Sciex, discusses the company’s collaboration with KCAS Bio and introduces their…