Posts by Dominic Warrino, Ph.D.
Dominic's role is to serve as scientific and technical advisor for both clients and internal teams for development, validation, and application of bioanalytical, immunogenicity, and biomarker methods for large molecule therapeutics. He has 20 years of experience developing and validating immunological assays for biotechnology and pharmaceutical companies.
 Blogs
Blogs
Pharmacodynamics (PD) is defined as the study of the biochemical and physiological effects of drugs and the mechanisms of their actions. Where pharmacokinetics looks at how the organism processes the drug, pharmacodynamics studies how…
 Blogs
Blogs
I have been studying Biological Markers (biomarkers) for over 25 years, but last 5 years they have exploded and become a key bioanalytical component to a drug’s success. Biomarkers are used to support drug…
 Blogs
Blogs
The long-term economic consequences of the COVID-19 virus are yet to be determined. However, short term it is having major impact on research & drug development so companies are adapting in order to survive.
 Blogs
Blogs
Biomarker is a broad term and can mean so many different things depending upon the scientific discipline. For Bioanalytical biomarker analysis – and specifically for CROs equipped with 9 different platforms to measure biomarkers and a cell culture suite with up to 19 color flow cytometry all under one roof…
 Blogs
Blogs
The bioanalytical landscape for support of biopharmaceuticals (biologics or large molecules) and biomarker testing is an ever changing map. The industry is currently focused on low level sensitivity, data integrity, and compliance with the…
 Blogs
Blogs
Protein characterization is a broad term describing the profile or fingerprint of a large molecule’s (or biologic’s) physical, chemical, and biological properties. Characterization of the protein will test the purity, activity, and the quantity…
 Blogs
Blogs
Adoption of technology has a life cycle that typically follows a bell-shaped curve. There are early adopters, early majority, late majority, and laggards. Adopting technology early comes with a risk, as not all technologies are widely adopted and many are very short lived. Adopting late also comes with a risk,…
 Blogs
Blogs
As the Bioanalytical support for drug development continues to grow, more and more scientists are requiring more complex assays. Two examples are cell based assays and what we refer to as live cell assays.
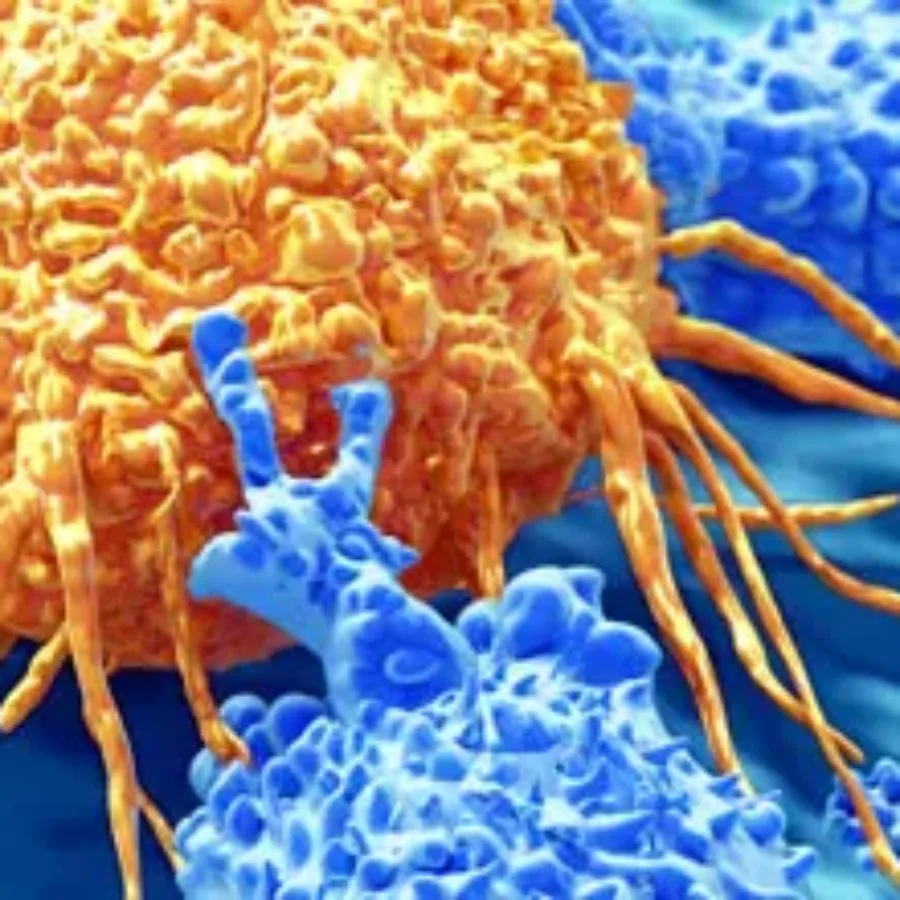 Blogs
Blogs
Immuno-oncology is a growing field. The goal is to augment the patient’s immune system to attack the cancer cells. The current lines of treatment for cancer are radiation, chemotherapy, and surgical resection. If these treatments do not work, physicians can seek alternative treatments, such as immune-oncology therapeutics. Two emerging immune-oncology…
 Blogs
Blogs
KCAS is the first GLP laboratory to qualify all 54 biomarkers offered as a V-Plex (Validated Plex) by MSD. The a la carte style allows for customization of panels that will fit the exact…
 Blogs
Blogs
Measuring new biotherapeutic modalities such as cellular therapies are not well defined in the regulatory landscape. Currently no white papers or guidelines are available to help navigate the murky waters of quantifying new modalities, which is why the AAPS PK focus group has set a course toward providing the field…
 Blogs
Blogs
Finding a laboratory to successfully transfer and GLP validate your PK method can be a challenge. Yes sure the big labs can do it, but if you are not a preferred provider you may find yourself working with the B team that doesn’t move as fast as you need, and…