Posts by Christine Bain, Ph.D.
Christine brings more than 25 years of experience in immunology and coordination of immune monitoring studies in the context of clinical trials in oncology and infectious diseases. She is now in charge of scientific affairs, from the development of internal R&D programs to the implementation of external collaborative projects with our partners.
 Blogs
Blogs
Originally developed to monitor B cells producing antigen- or pathogen-specific antibodies, the ELISpot assay is widely used for evaluating vaccine-induced wanted immunogenicity. However, thanks to its high sensitivity, it is also a valuable tool for investigating unwanted immunogenicity, expanding its applications beyond traditional vaccine research. Early…
 Blogs
Blogs
What Are Ligand Binding Assays (LBAs)? Ligand binding assays (LBAs) have been our core activity for decades. LBAs are commonly used to measure interactions between two proteins, a ligand and its receptor, a monoclonal antibody (mAb) and its target, or a biologic and Anti-Drug Antibodies (ADA). Applications of…
 Blogs
Blogs
What is immunogenicity? Immunogenicity refers to the ability of a substance, such as a New Biological Entity (NBE) or a vaccine, to provoke an immune response when administered in the body. The recognition of the substance as foreign triggers an immune response, which involves the production of antibodies and the…
 Blogs
Blogs
Remember that childhood excitement the night before Christmas? Too excited to sleep, knowing something you wished for was finally within reach? That’s exactly the spirit fueling our teams in Lyon as we approach the end of June. We’re eagerly awaiting the arrival of a powerful new platform at our…
 Blogs
Blogs
Biomarkers (BMKs) have become fundamental tools in drug development, accelerating and optimizing targeted therapeutic innovation. As a bioanalytical CRO with over 15 years of experience in biomarker analysis, we’ve seen firsthand how the strategic integration of biomarkers can accelerate drug programs, help meet evolving regulatory standards,…
 Blogs
Blogs
We were happy to participate in the AD/PD™ 2025 International Conference on Alzheimer’s and Parkinson’s Diseases and related neurological disorders, held from April 1 to 5, 2025, in Vienna, Austria. The conference was a valuable opportunity to connect with experts, share our experience, and gain insights…
 Blogs
Blogs
Initially developed to assess the frequency of circulating antigen-specific Antibody-Secreting Cells (ASC), ELISpot has become a vital tool for quantifying antigen-reactive T-cells by measuring secreted immune mediators such as cytokines or key molecules involved in cell-mediated cytotoxicity. Compared to other assays for monitoring cell-mediated immunity (CMI), such as…
 Posters & Papers
Posters & Papers
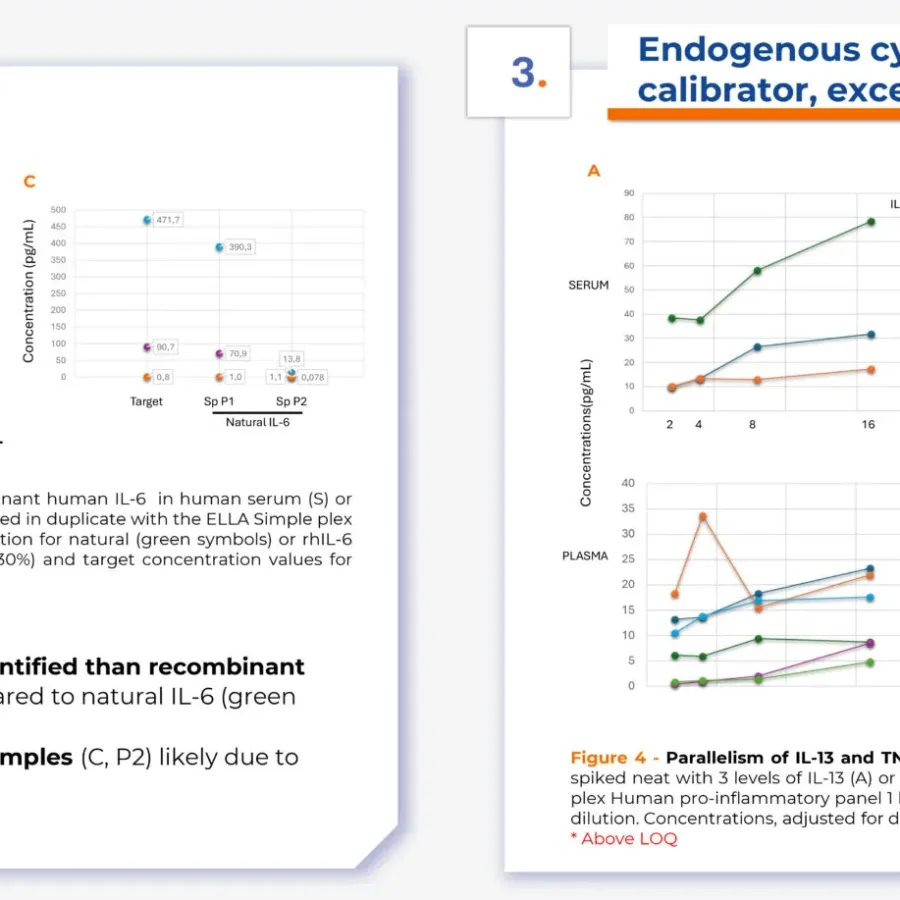
Discover innovative insights into bioanalytical method development with our poster, presented by Christine Bain, Ph.D., at ECCO 2025. This work explores the creation and application of reference samples containing endogenous immune mediators—such as cytokines and chemokines—to effectively evaluate the performance of bioanalytical methods. Download the poster now to learn more…
 Blogs
Blogs
Last month, Elodie, Magali, and I found ourselves in Lisbon—not for a spring getaway (though the weather was a delightful preview of the season) and certainly not for ultra-trail training (although the city’s hills did give our calves a solid workout!). Instead, we were there for EIP, an event…
 Blogs
Blogs
What is a generic PK assay? A generic pharmacokinetic (PK) assay, also referred to as a universal PK assay for New Biologic Entities (NBE), is used to measure circulating human biotherapeutics—primarily antibody-based—post-administration across various non-human matrices. These assays are particularly beneficial when drug-specific reagents, such as anti-idiotype antibodies, are…
 Blogs
Blogs
The Role of Pharmacokinetics (PK) in Drug R&D Pharmacokinetics (PK) is defined as the study of the fate of a new therapeutic entity (NTE) after it is administered into the body of animals or humans. It involves understanding how the drug is absorbed, distributed, metabolized, and eliminated (ADME). Essentially, PK…
 Blogs
Blogs
Charcot, Gehrig, Hawking: A Journey Through ALS When you hear the names Charcot, Gehrig, and Hawking, what comes to mind? These names are all linked by a shared thread: Amyotrophic Lateral Sclerosis, or ALS. ALS, also known as Charcot disease, is named after Jean-Martin Charcot, a neurologist from the Pitié-Salpêtrière…