Bioanalytical CRO Excellence
Welcome to KCAS Bio, your global bioanalytical CRO partner with the expertise, capacity and flexibility sponsors require.

CRO for preclinical and clinical bioanalysis
KCAS Bio is a leading bioanalytical contract research organization (CRO) with a proven record of advancing drug development worldwide. We have provided bioanalytical development support for more than 315 approved drugs on the market, developed over 6,200 proprietary and non-proprietary assays, and successfully completed more than 20 FDA audits with no major findings.
As a top bioanalytical CRO lab, we continually invest in the latest instrumentation and combine this technical expertise with a consultative approach, ensuring our clients’ goals are achieved with confidence. Our teams also bring specialized expertise in advanced modalities, allowing us to support the evolving needs of modern drug development. By consistently benchmarking and optimizing our operations, we help scientific innovators and investors move forward with greater certainty.
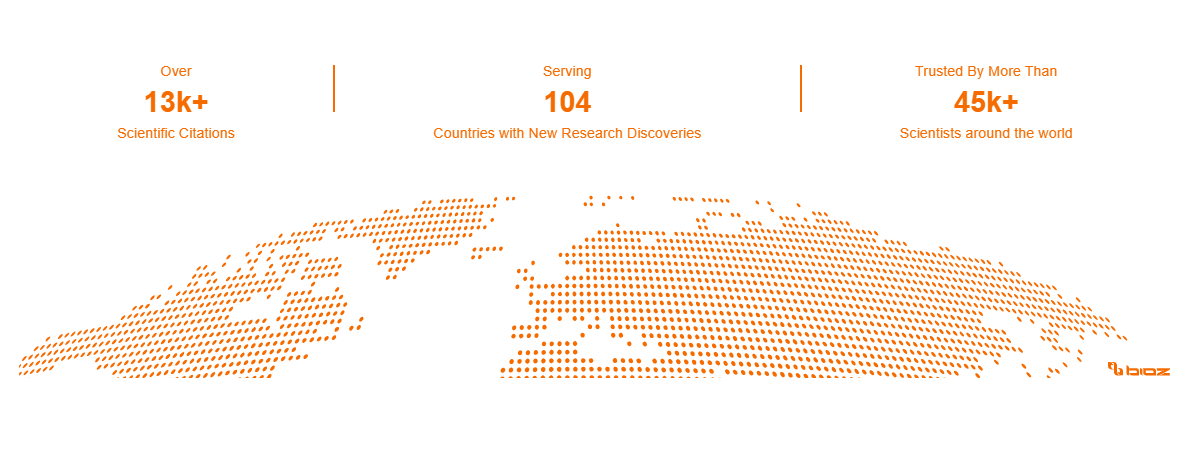
With lab facilities across the US and Europe, along with a strategic alliance in Australia, we provide global sponsors with comprehensive services including bioanalytical, biomarker, immunogenicity, cellular and molecular assay capabilities, as well as clinical kitting and sample management.
Did you Know? We have Harmonized Spectral Flow Cytometry on a Global Scale!
KCAS Bio is the only bioanalytical laboratory with globally harmonized Cytek spectral flow cytometers, with sites in the US, EU and Australia.


![]()



Global biomarker and bioanalytical CRO lab
KCAS Bio is an established bioanalytical CRO with over 45 years of experience. We understand the human and financial impact of drug development delays. Through our unique approach to capacity planning, we combine the right experts with the right instrumentation to keep development moving smoothly. Our clients consider us a reliable partner because we strive to minimize downtime, avoid delays, and keep lead times as short as possible.

Find exactly what you’re looking for
Search for specific services, platforms, instrumentation, or scientific insights.
What our clients say
We consistently earn our clients’ trust based on our reputation for transparency, delivering error-free data and keeping our promises.
KCAS Bio scientific insights
 Podcasts
Podcasts
In episode #97 of “The Weekly Bioanalysis” podcast, John and Dom focus on a review of 2025 drug approvals, revealing a surprisingly strong year for small molecules despite long-standing predictions of their decline. Of the 53 FDA approvals, 31 were small molecules, with additional complexity coming from ADCs and RNAi…
 Blogs
Blogs
Developing anti-drug antibody (ADA) assays for therapeutic peptides can be complex, particularly when the molecule is small, endogenous-like, and when no positive control exists. This article describes the stepwise development of a screening and confirmatory ADA assay for a 47-amino-acid peptide targeting a receptor involved in inflammation in a…
 Podcasts
Podcasts
In episode 28 of “The Conversational Flow”, our hosts, Adam and Brian, discuss why standardization is essential for credible flow cytometry data at scale. They walk through the biggest sources of variability – reagents and lots, sample handling and shipping time, anticoagulants and processing choices (whole blood vs. PBMCs, fresh…
Advancing great people and great science
We make nurturing new talent a strategic priority, so that teams are dynamic and constantly improving. We provide a supportive environment for continuous learning and training where you can become your best.


Tell us how we can help with your project
We've earned our reputation for delivering reliable, error-free data. We understand the importance of speed, flexibility, and consistency and only make promises we can keep.