 Blogs
Blogs
What Are Ligand Binding Assays (LBAs)? Ligand binding assays (LBAs) have been our core activity for decades. LBAs are commonly used to measure interactions between two proteins, a ligand and its receptor, a monoclonal antibody (mAb) and its target, or a biologic and Anti-Drug Antibodies (ADA). Applications of…
 Blogs
Blogs
In a bioanalytical CRO, method development is a critical step to ensure reliable and meaningful data that meets the client’s requirements. At our KCAS Bio – Lyon site, our method development team specializes in designing and optimizing ligand-binding assay (LBA) methods tailored to our clients’ specific objectives. While…
 Blogs
Blogs
What is immunogenicity? Immunogenicity refers to the ability of a substance, such as a New Biological Entity (NBE) or a vaccine, to provoke an immune response when administered in the body. The recognition of the substance as foreign triggers an immune response, which involves the production of antibodies and the…
 October 7
- October 10
October 7
- October 10
KCAS Bio will be participating in the Immunogenicity & Bioassay Summit 2025, taking place October 7-10 in Alexandria, VA. This annual summit, hosted by the Cambridge Healthtech Institute, brings together leaders in immunogenicity assessment, bioassay development, and translational science to explore emerging strategies in drug development. With focused tracks on…
 Blogs
Blogs
Remember that childhood excitement the night before Christmas? Too excited to sleep, knowing something you wished for was finally within reach? That’s exactly the spirit fueling our teams in Lyon as we approach the end of June. We’re eagerly awaiting the arrival of a powerful new platform at our…
 Posters & Papers
Posters & Papers
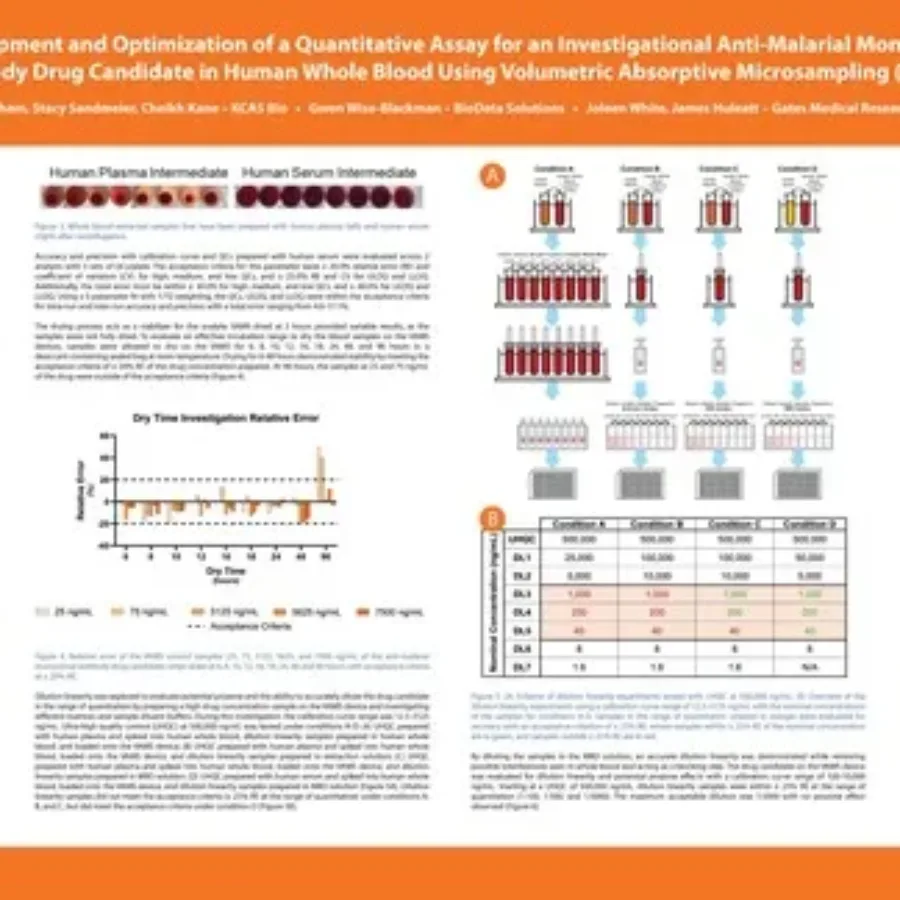
Discover in this poster presented by Jessica Pham at WRIB 2025 on the “Development and Optimization of a Quantitative Assay for an Investigational Anti-Malarial Monoclonal Antibody Drug Candidate in Human Whole Blood Using Volumetric Absorptive Microsampling (VAMS)”. If you have any questions about these services or any others offered by…
 Blogs
Blogs
Initially developed to assess the frequency of circulating antigen-specific Antibody-Secreting Cells (ASC), ELISpot has become a vital tool for quantifying antigen-reactive T-cells by measuring secreted immune mediators such as cytokines or key molecules involved in cell-mediated cytotoxicity. Compared to other assays for monitoring cell-mediated immunity (CMI), such as…
 Blogs
Blogs
ADCs represent a promising class of targeted therapies, and understanding the intricacies of their analysis is crucial for their successful development. In this blog, we address common questions to guide you through the various considerations involved in ADC research and testing. We will cover key topics related to the…
 Blogs
Blogs
Antibody-drug conjugates (ADCs) have been conventionally developed for a wide range of oncological applications since the first ADC approval in 2000 by the FDA. Typically, an ADC consists of three main components: antibody, linker conjugate, and therapeutic drug (payload). The mechanism of an ADC involves the antibody binding to a specific…
 Posters & Papers
Posters & Papers
Discover in this poster presented by Fabien Lavocat at EBF Open Sympoisum 2024 on “Comparative Evaluation of Ligand Binding Assay Platforms for Biomarker Quantification: Critical Considerations for Ensuring Data Quality and Project Success”.
 Posters & Papers
Posters & Papers
Please download this poster, “Automating Selection for Incurred Sample Reanalysis: A Meta-Analytical-Based Algorithm for Error Reduction”
 Blogs
Blogs
A pharmacokinetic (PK) assays evaluate how the body affects a specific substance after administration which includes: absorption, biodistribution, metabolism, and excretion. Preclinical (or non-clinical) PK assays play a crucial role in drug development and typically focus on assessing drug safety and maximum tolerable dose. For the development of pre-clinical PK…