 Blogs
Blogs
Hybrid LC-MS/MS is a technique that combines an affinity capture step with LC-MS/MS detection. It typically requires only one antibody, in contrast to conventional ligand-binding assays (LBAs), which usually need two. This approach leverages the combined selectivity of affinity extraction and the analytical power of tandem mass…
 Posters & Papers
Posters & Papers
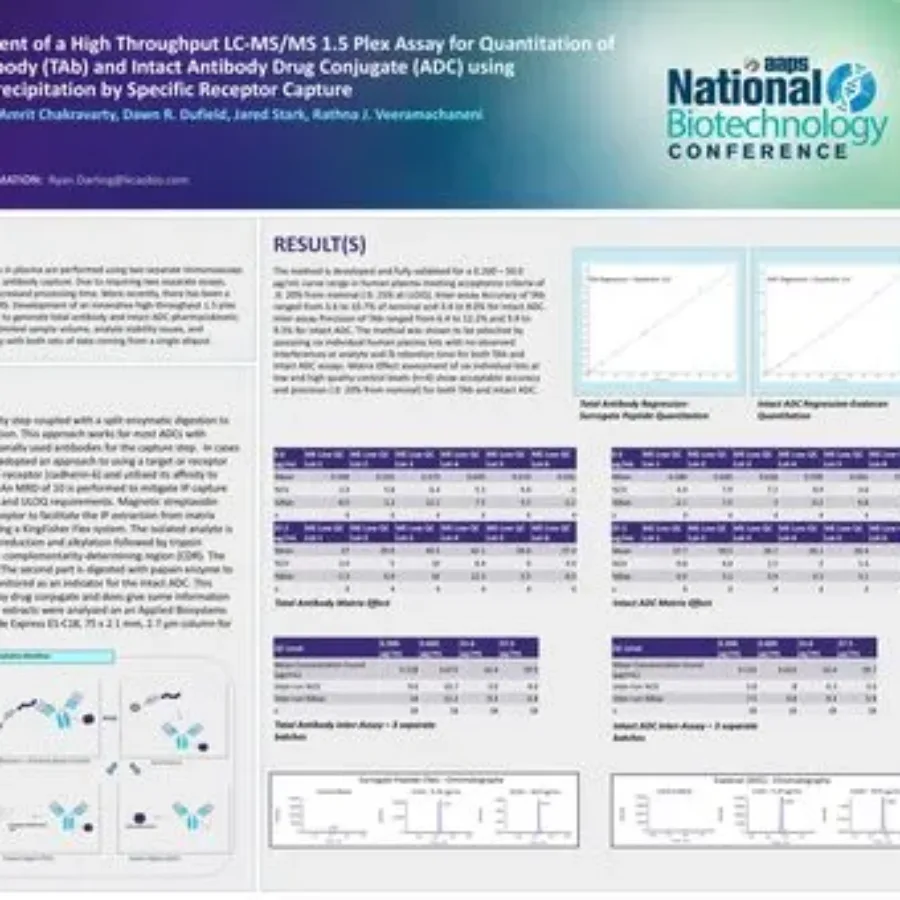
Discover in this poster presented by Ryan Darling at the AAPS National Biotechnology Conference 2025 on the use of a hybrid LC-MS/MS approach for the quantitation of total antibody TAb and intact Antibody Drug Conjugate (ADC). HybridLC-MSMSAssayforADCQuantitation_KCASBioDownload If you have any…
 Posters & Papers
Posters & Papers
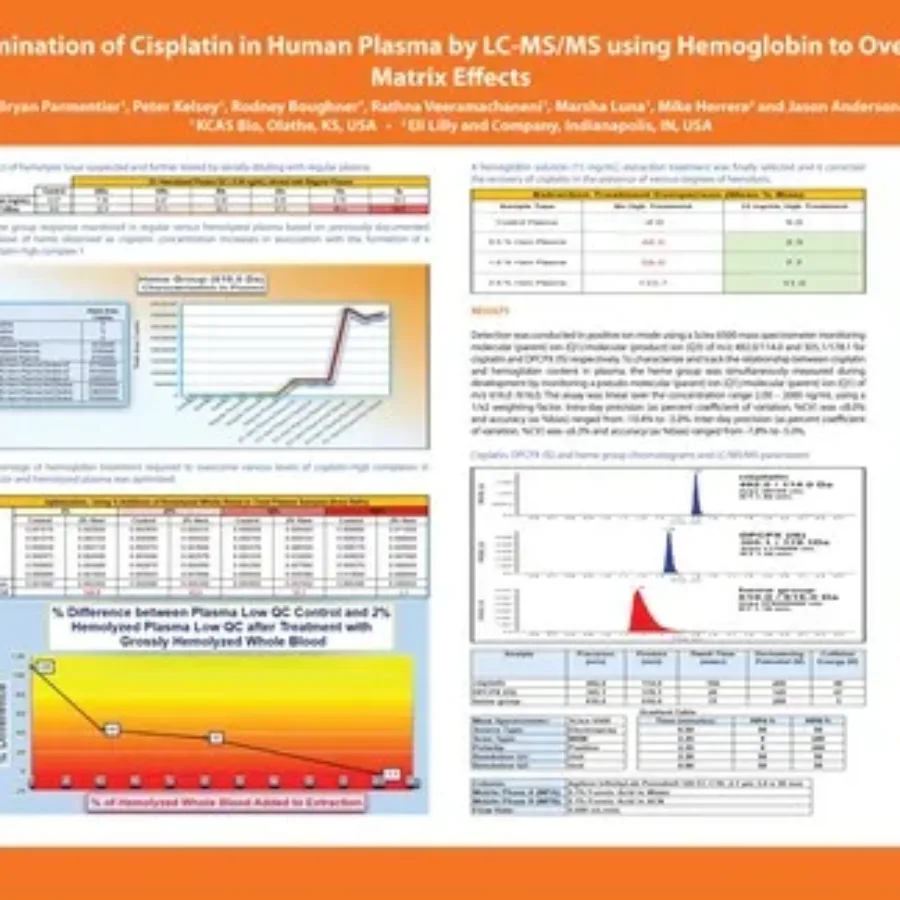
Discover in this poster presented by Bryan Parmentier at WRIB 2025, our work for the sponsor Lilly on the “Determination of Cisplatin in Human Plasma by LC-MS/MS using Hemoglobin to Overcome Matrix Effects“. Cisplatin In Human Plasma By LC-MS/MS | KCAS BioDownload If…
 Blogs
Blogs
ADCs represent a promising class of targeted therapies, and understanding the intricacies of their analysis is crucial for their successful development. In this blog, we address common questions to guide you through the various considerations involved in ADC research and testing. We will cover key topics related to the…
 Blogs
Blogs
Antibody-drug conjugates (ADCs) have been conventionally developed for a wide range of oncological applications since the first ADC approval in 2000 by the FDA. Typically, an ADC consists of three main components: antibody, linker conjugate, and therapeutic drug (payload). The mechanism of an ADC involves the antibody binding to a specific…
 Blogs
Blogs
Dose Formulation Analysis is an essential step in regulated nonclinical studies. Robust analytical methods, rapid turnaround, and efficient communication helps ensure the dose form results for your GLP studies are delivered on time. At KCAS Bio, our scientific expertise allows us to develop methods that achieve…
 Blogs
Blogs
Oligonucleotide therapeutics have recently gained popularity as noted by recent increases in regulatory approvals of oligonucleotides as drugs, novel liquid nanoparticle delivery approaches, and on-target specificity. In the development of bioanalytical assays, there are many challenging aspects to consider when quantitating oligonucleotides, such as non-specific binding, stability, efficient ionization, sequence…
 Blogs
Blogs
When you examine how to develop and validate/qualify a Biodistribution assay by PCR…
 Webinars
Webinars
As the pharmaceutical industry continues to evolve, so does the technology behind drug development. One of the most groundbreaking advancements in bioanalysis is Hybrid LC-MS/MS technology, which is rapidly becoming the new standard for analyzing Antibody Drug Conjugates (ADCs). KCAS Bio is proud to be at the forefront of this…
 Blogs
Blogs
2020 was an odd year for everyone. In what felt like just days, it seemed everything had changed and the rules were being rewritten almost daily about how we would all be engaging with one another. We went from racking up travel, hotel stays and airmiles while visiting our customers…
 Blogs
Blogs
The age of therapeutic conjugation is upon us! Bioanalysis for support of next-generation Antibody Drug Conjugates (ADCs) and Antibody siRNA Conjugates (ARCs) have exploded recently due to the efficacy and safety that these therapies offer for immuno-oncology, rare diseases, vaccines, and potentially many other diseases. Recently, we have seen the…
 Blogs
Blogs
Those of you attending the 18th annual WRIB meeting in San Antonio, TX are invited to attend a poster presentation being given by members of the KCAS Bio team: Jack Rogers, Jessie Allen, Yoka Thomas, and Cheikh Kane. This project developed and characterized a novel algorithm for automated ISR selection…