 Blogs
Blogs
KCAS Bio SAS (Active Biomarkers SAS) based in Lyon France, a full-service bioanalytical and biomarkers solutions provider is very pleased and proud to share that it successfully opened a new Good Laboratory Practice (GLP) Test Facility to provide GLP-grade bioanalytical methods in the context of preclinical studies. Since November 2023,…
 Blogs
Blogs
In the scientific field, the often neglected fifth sense, the sense of smell, contributes to broadening our understanding of various phenomena, particularly in the field of biomarkers.Here, we will guide you along our lines of thought on the importance of our olfactory sense, and how it contributes to scientific exploration…
 May 15
- May 16
May 15
- May 16
Knowledge for Growth (KfG) is Europe’s premier life sciences conference, held annually in Antwerp, Belgium. This year’s edition celebrates the industry’s advancements with a hybrid format, catering to both in-person and virtual attendees. The program features captivating talks, scientific sessions on cutting-edge topics like gene editing and personalized medicine, and…
 May 4
- May 8
May 4
- May 8
CYTO 2024, hosted by the International Society for the Advancement of Cytometry (ISAC), is the premier annual congress dedicated to the science and engineering of cytometry. This prestigious event, scheduled for later this year, serves as a global platform for researchers, clinicians, and industry leaders to convene, share knowledge, and…
 April 22
- April 23
April 22
- April 23
Swiss Biotech Day is a premier European biotechnology conference that brings together industry professionals from all over the world. The 2024 event will be held on April 22nd and 23rd at the Congress Center Basel in Switzerland. The conference offers a variety of opportunities for attendees to learn about the…
 April 22
- April 24
April 22
- April 24
The European Immunogenicity Platform (EIP) is a central meeting place for scientists and biopharmaceutical companies in Europe. The goal of the EIP is to improve knowledge and expertise in immunogenicity. This is achieved through collaboration between scientists and industry experts. The EIP works with companies, institutes, and professionals involved in…
 April 9
- April 11
April 9
- April 11
The Cell & Gene Meeting on the Med is a premier European conference focusing on advanced therapy medicinal products (ATMPs). ATMPs are a revolutionary class of treatments that involve modifying a patient’s cells to fight disease. This conference unites researchers, developers, manufacturers, and other stakeholders from around the world who…
 Videos
Videos
Clients typically will come to us saying that they have a therapy, and it is going to address these targets, this disease, to treat certain patients. They want to know how we can best understand what’s happening and how the immune system will respond. We need to know what cells…
 Videos
Videos
One of the coolest things we’re doing here at KCAS Bio is definitely validating new instruments and new software. We start off with the Cytek systems of course, and then we’re adding in FCS Express, we’re applying both of those tools ’cause that’s really all they are at the…
 Videos
Videos
KCAS has recently moved from a space that was extremely tight – even for the operations we had at the time – to an enormous new modern lab building that is purpose-built for the type of work that allows all of our workflows to be more efficient, more scalable,…
 Blogs
Blogs
For the last decade or so, the outsourcing of crucial research functions by pharmaceutical and biotechnology companies has become increasingly common and has resulted in the rapid growth of the Contract Research Organization (CRO) industry. But the selection of the best CRO partner to work on your critical research programs…
 Podcasts
Podcasts
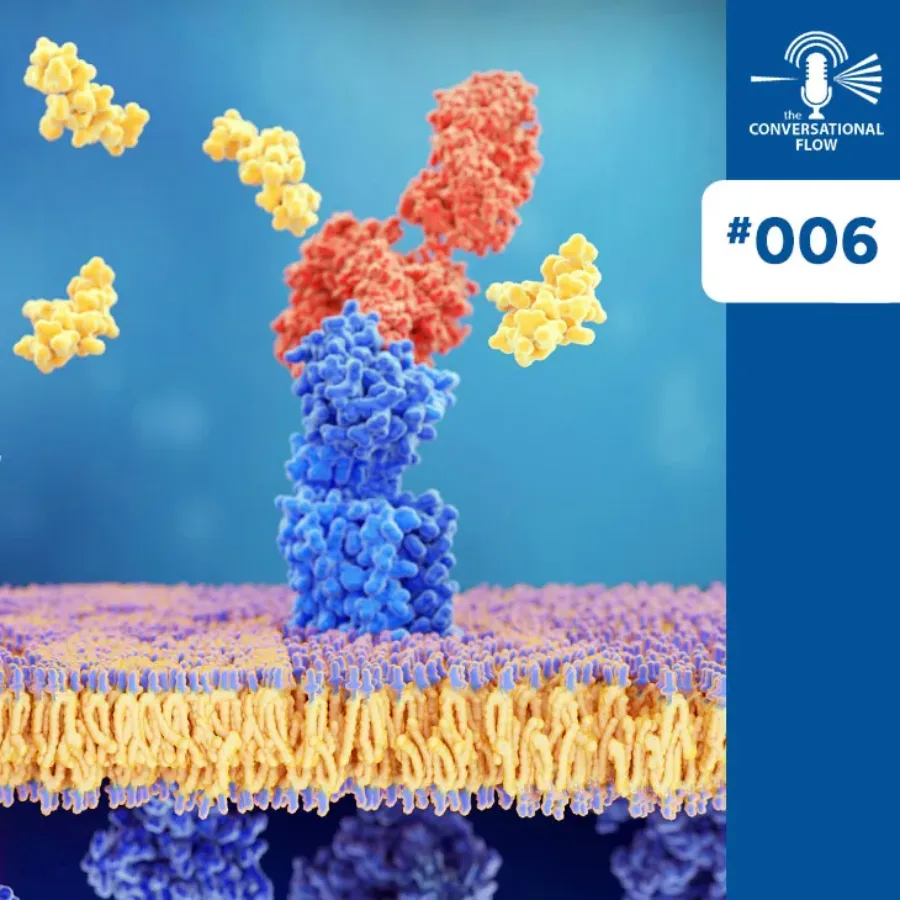
The newest episode of “The Conversational Flow” has Brian and Adam talking about the concepts around Receptor Occupancy and all the deceptively simple questions people often have about them, as well as the important but sometimes difficult answers to those questions. They talk about the biology of receptors and the…