 Blogs
Blogs
The FDA has regulated cigarettes, smokeless and loose tobacco since 2009 under the Tobacco Control Act. In 2016, the FDA finalized a rule for the regulation of all tobacco products, making it illegal to sell e-cigarettes, cigars and hookah tobacco to anyone under 18 and requiring retailers to check photo…
 Blogs
Blogs
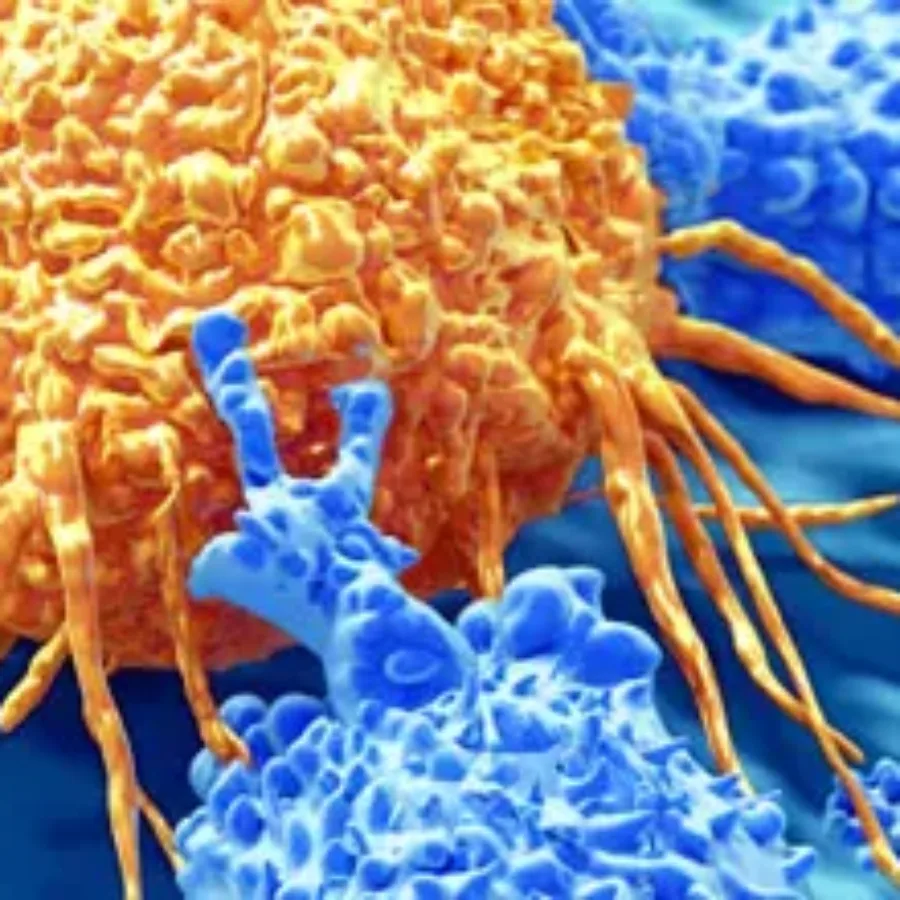
Immuno-oncology is a growing field. The goal is to augment the patient’s immune system to attack the cancer cells. The current lines of treatment for cancer are radiation, chemotherapy, and surgical resection. If these treatments do not work, physicians can seek alternative treatments, such as immune-oncology therapeutics. Two emerging immune-oncology…
 Blogs
Blogs
Once again we find ourselves wrapping up our 2017 goals and and tallying the year’s performance. Simultaneously, we are filled with excitement looking forward to the New Year and to the promises it will bring to the pharmaceutical industry and the CRO community. According to Reuters, 2017 was one of…
 Blogs
Blogs
KCAS is the first GLP laboratory to qualify all 54 biomarkers offered as a V-Plex (Validated Plex) by MSD. The a la carte style allows for customization of panels that will fit the exact…
 Blogs
Blogs
Several important classes of drugs are inherently unstable in biological fluids. Examples include alkylating agents, cytostatic nucleosides, drugs with ester, aldehyde, thiol, nitroxyl or lactone functional groups, and small molecule compounds with unstable metabolites, such as acylglucuronides. The rate at which an analyte degrades in blood, other biological fluids, or…
 Blogs
Blogs
The 505(b)(2) drug development path, in contrast to traditional development of a new, never been approved drug (described under 505(b)(1) of the Federal Food, Drug, and Cosmetic Act) or ANDA (described under Section 505(j) of the Act) for a generic drug that is no longer under patent…
 Blogs
Blogs
Designing a tissue assay in accordance to the FDA bioanalytical method validation (BMV) guidance involves three key questions. First, can control tissue be easily obtained in bulk quantities or should a surrogate tissue approach be considered? Second, what sample processing procedures should be considered relative to the known stability of…
 Blogs
Blogs
Measuring new biotherapeutic modalities such as cellular therapies are not well defined in the regulatory landscape. Currently no white papers or guidelines are available to help navigate the murky waters of quantifying new modalities, which is why the AAPS PK focus group has set a course toward providing the field…
 Blogs
Blogs
In pursuit of KCAS’ Mission to provide reliable and defendable data, quality is one of our core values. It is the responsibility of every employee to provide an always improving, high-level performance in all we do. Our independent Quality Assurance Unit (QAU) assures management that Operations is performing in conformance…
 Blogs
Blogs
Immunogenicity, why do we keep talking about it? Immunogenicity can, in the simplest of terms, be described as a subject’s ability to generate antibodies specific to the dosed protein therapeutic. While stating it this way understates the complexity of the immune system and the methods needed to detect and characterize…
 Blogs
Blogs
Surprisingly, there are a number of drug development companies that do not obtain stable isotope-labeled internal standards (SIL-IS) for their bioanalytical methods. Instead they settle for using a surrogate internal standard (a compound that closely resembles the measured analyte) which can lead to unreliable data and be detrimental to a…
 Blogs
Blogs
What you don’t know about how an off-the-shelf Biomarker kit is evaluated and qualified can significantly compromise your biomarker data. Running the kit using the manufacturer’s instructions without systematically testing the accuracy/precision (A/P), range of quantification (ROQ), dilutional linearity (DL), stability, and selectivity can jeopardized the interpretation of the results.