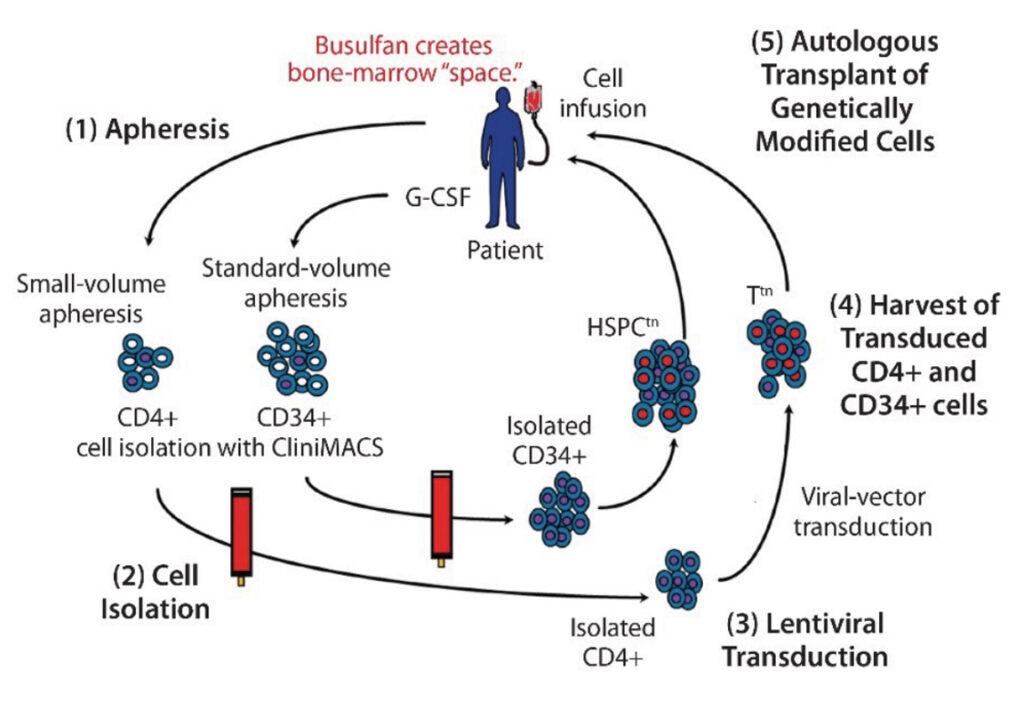
With the extensive advances in technologies like CRISPR and CAR-T, cell and gene therapy has grown to become a viable way for treating Cancer as well as other diseases. Our team has over 100+ years of collective expertise in molecular services using qPCR and ddPCR for support of pre-clinical and clinical trials in a wide range of species and matrices. Our new state of the art 70,000 square ft facility has a unidirectional workflow that includes 2 dedicated preamplification areas (area 1 reagent preparation & storage and area 2 sample preparation & extraction) and a dedicated postamplification area (area 3 amplification and manipulation) for support of molecular services. We are ready to provide reliable and defendable results for your bioanalytical molecular needs.
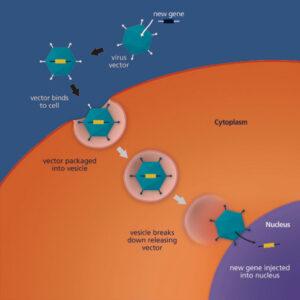
Gene Therapy uses genetic material to modify the function of patient cells in the different gene therapy treatments: Gene replacement, Gene addition, Gene inhibition and Gene editing. Cell Therapy is the infusion or transplantation of whole cells from a patient and then in some cases, modifying the cell genome before re-injecting them back into the patient. Here at KCAS, we have a new facility designed specifically handle both of these types of therapies analysis in graduated sterile rooms.
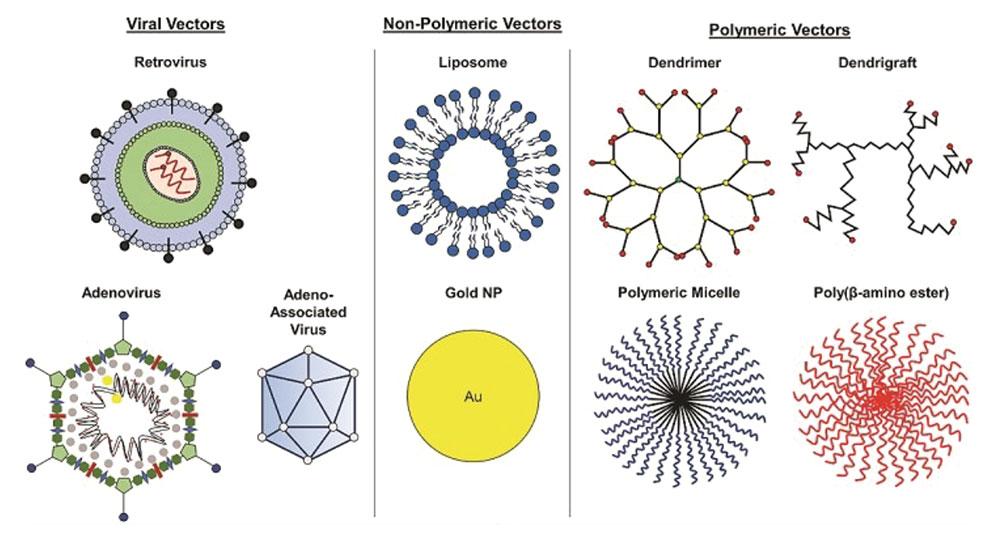
Vector Screening:
• Vector composition analysis. LBA-LC-MS/MS, LBA
• Immunogenicity and Modifications made to the viral vectors, transgene, or other types of Vectors; Modified LBA immunogenicity
• Hybrid LBA-LC-MS/MS to Identify structure modifications of Vector
Gene Payload:
• PK with qPCR, ddPCR and LBA assays
• LBA Cell-based Nab assays based on Vectors and cell type etc.
• Biodistribution with qPCR, ddPCR, LBA assays
• PD Markers with Luminex, qPCR, ddPCR, Flow Cytometry, ELISPOT
Multiple Services to Address all Aspects of Cell Therapy:
• PK with Flow Cytometry, qPCR, ddPCR
• Biodistribution with Flow cytometry, qPCR, ddPCR
• Immunogenicity with Flow Cytometry, LBA cell-based Assays (Nab)
• Pharmacodynamic Markers with Luminex, qPCR, ddPCR, flow Cytometry (High Parameter flow cytometry), ELISPOT
Platforms & Technology
The KCAS team is highly knowledgeable with decades of hands-on experience. We work with our Cell and Gene Therapy customers to move their drug products from Discovery through Development, and into the clinic using a number of state-of-the-art platforms and technologies.
Our team has hands on experience and knowledge of the intricacies of the drug development process for quantification of the gene, vector and/or target cell. Our Scientists know the GxP regulatory guidances and will collaborate with you and your team in developing bioanalytical methods for for support of cell and gene therapies.
If you have any questions for our KCAS team about Cell & Gene Therapy, or any of our other services, please use the form below to contact them.