Posts by KCAS Bio
 Posters & Papers
Posters & Papers
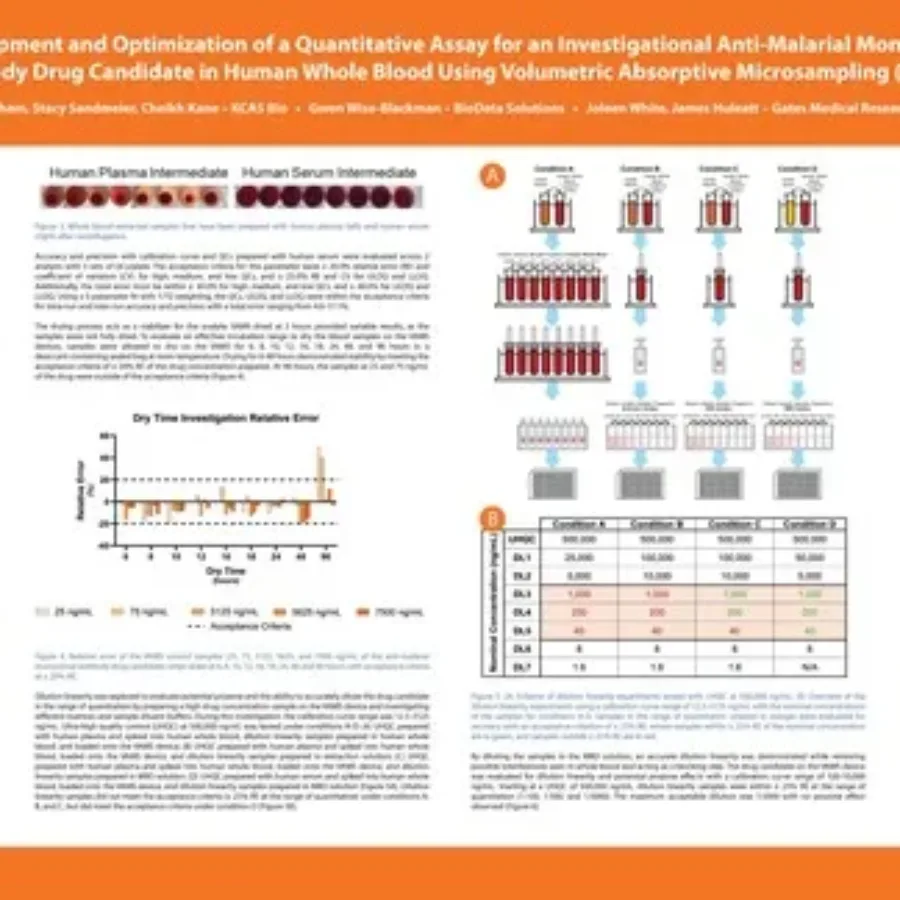
Discover in this poster presented by Jessica Pham at WRIB 2025 on the “Development and Optimization of a Quantitative Assay for an Investigational Anti-Malarial Monoclonal Antibody Drug Candidate in Human Whole Blood Using Volumetric Absorptive Microsampling (VAMS)”. If you have any questions about these services or any others offered by…
 Posters & Papers
Posters & Papers
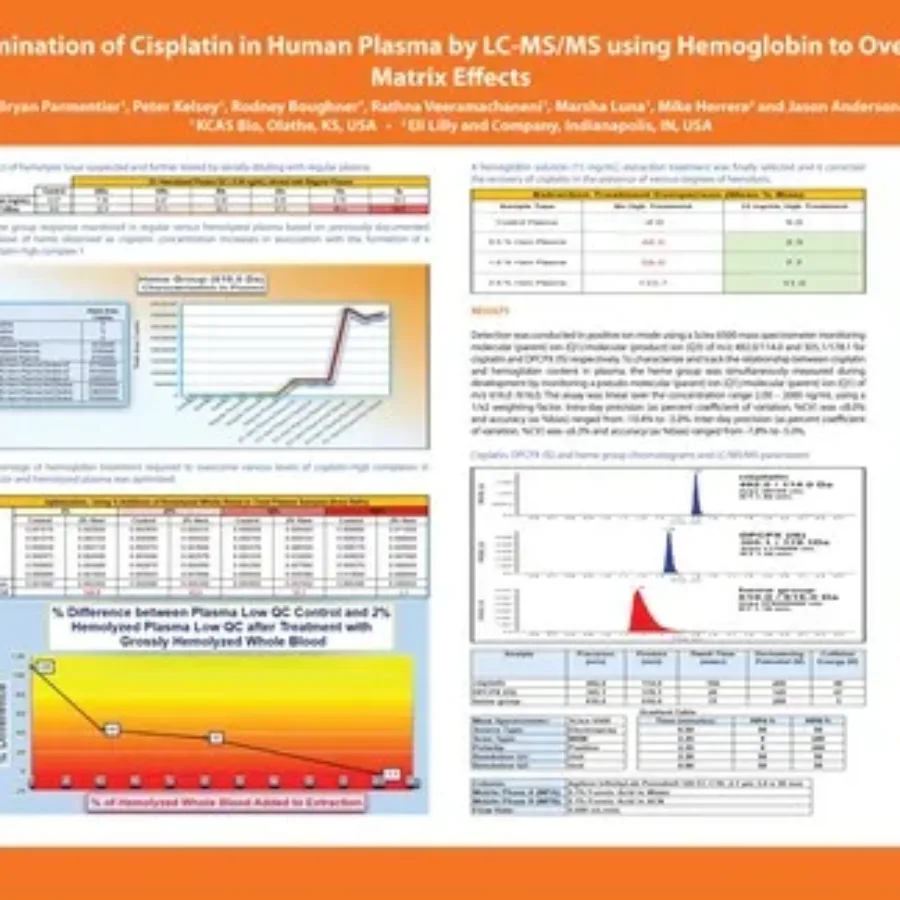
Discover in this poster presented by Bryan Parmentier at WRIB 2025, our work for the sponsor Lilly on the “Determination of Cisplatin in Human Plasma by LC-MS/MS using Hemoglobin to Overcome Matrix Effects“. If you have any questions about these services or any others offered by KCAS Bio, please feel…
 Posters & Papers
Posters & Papers
Discover in this poster presented by David Ambrose at WRIB 2025, our work on the Global Harmonization of a Validated 15-Color Pan-Leukocyte Spectral Flow Cytometry Panel for Human Whole Blood in Support of Translational and Clinical Applications. If you have any questions about these services or any others offered by…
 Blogs
Blogs
Modern immunology research requires high-complexity panels available wherever the patients are located. Deploying advanced flow cytometry instruments and panels across multiple global sites is therefore a transformative step in harmonizing immunological research and clinical trials. KCAS Bio has worked closely with Cytek to make this a reality by validating the…
 Blogs
Blogs
The Society of Toxicology’s SOT 2025 meeting in Orlando, Florida, held from March 20-22, was a unique gathering that highlighted both the ongoing challenges and the evolving opportunities within the toxicology and bioanalytical fields. As a Bioanalytical CRO providing critical dose formulation testing and toxicology studies…
 News
News
Press Release • Mar 26, 2025 PALO ALTO, Calif., March 26, 2025 (Newswire.com) – Bioz, Inc., the leader in citation management, is highlighting its partnership with KCAS Bio, a trusted provider of bioanalytical and biomarker services. Through the integration of a Bioz Content Hub, KCAS…
 webinars
webinars
As the field of biotherapeutics rapidly evolves, the development of advanced conjugated therapies such as Antibody-Drug Conjugates (ADCs), Antibody-RNA Conjugates (ARCs), siRNA/oligos, and antibody-peptide conjugates has gained significant momentum. These next-generation therapeutics offer promising efficacy and safety profiles for treating various conditions, including cancer, rare diseases, and in vaccine development.
 Blogs
Blogs
Polymerase Chain Reaction (PCR) has revolutionized molecular biology by enabling the rapid and precise amplification of DNA sequences. Since its invention by Kary Mullis in the 1980s, PCR has become an indispensable tool in both research and diagnostic applications. From identifying genetic disorders to detecting infectious diseases, PCR’s versatility has…
 Blogs
Blogs
KCAS Bio is excited to announce its participation in the upcoming 19th Workshop on Recent Issues in Bioanalysis (WRIB), taking place April 7-11, 2025, in New Orleans, Louisiana. As a long-standing attendee and active contributor at WRIB, KCAS Bio is proud to continue its tradition of…
 Blogs
Blogs
When it comes to developing new therapies for inflammatory bowel diseases (IBD), having robust and reliable bioanalytical support is crucial. From the early stages of drug discovery to Phase III clinical trials, our comprehensive bioanalytical solutions are designed to enhance and accelerate your drug development journey in a GCP…
 Blogs
Blogs
ADCs represent a promising class of targeted therapies, and understanding the intricacies of their analysis is crucial for their successful development. In this blog, we address common questions to guide you through the various considerations involved in ADC research and testing. We will cover key topics related to the…
 Blogs
Blogs
Understanding the intricate relationship between drugs and the human body is crucial for effective medical treatments. Two fundamental concepts in pharmacology – pharmacokinetics and pharmacodynamics – play pivotal roles in this understanding. This blog post will delve into these concepts, exploring their differences and significance in drug development and clinical…