 Blogs
Blogs
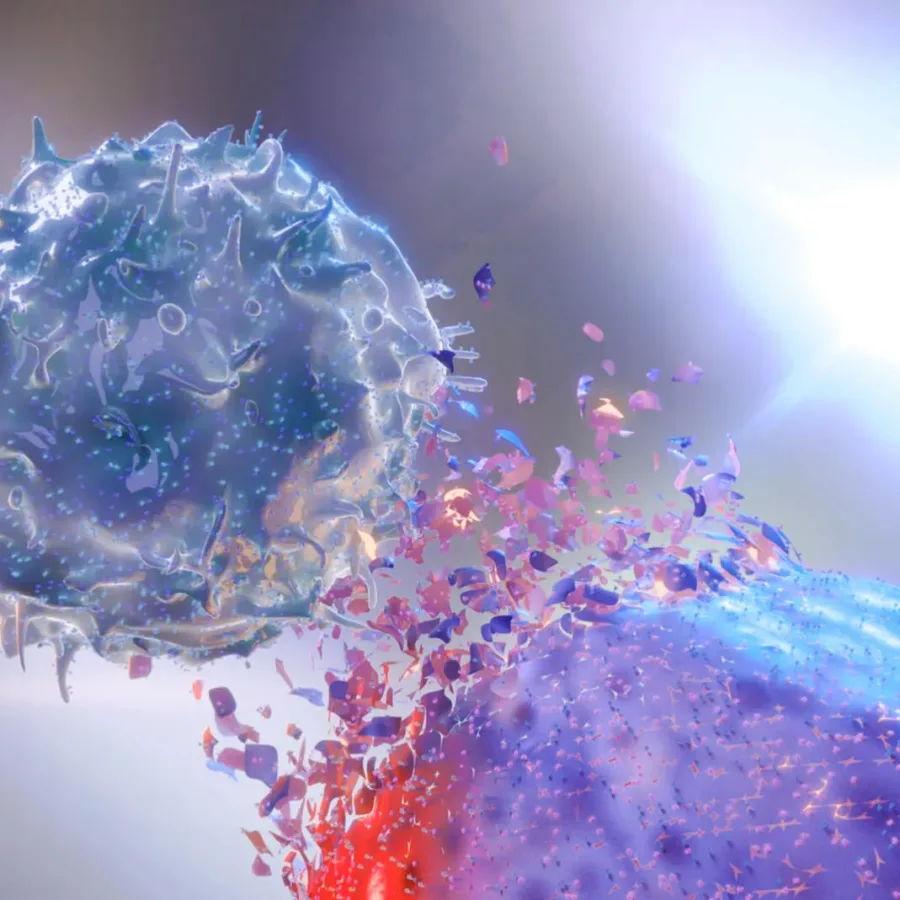
What is the primary role of Natural Killer (NK) cells? Natural killer (NK) cells are the predominant innate immune cells that mediate anti-tumor and anti-viral responses, and therefore possess good clinical utilization (Abel et al. 2018). Natural killer cells comprise 10–15% of peripheral blood lymphocytes and classically display a half-life of approximately 7–10 days in the circulation (Moretta et al. 2000).
 Blogs
Blogs
It was early March 2020, the tendons in my right hand were swollen and throbbing, and I was seeking to learn more about off-the-bench careers at CROs and finally retire from pipetting. A few…
 Blogs
Blogs
The Department of Health and Human Services (HHS) has issued long awaited, comprehensive update on how medical research should be conducted. The Common Rule, 45 CFR 46 subpart A, issued 26 years ago and established basic protections for human subjects in research conducted by or supported by the HHS and…