Antibody Drug Conjugates
At KCAS Bio, we leverage our bioanalytical expertise to support the development of next-generation ADCs, providing comprehensive services that ensure the efficacy and safety of these innovative treatments.

Advancing Antibody–Drug Conjugates: Precision Bioanalytical Services for Every Development Stage
KCAS Bio supports the development and characterization of Antibody–Drug Conjugates (ADCs) by providing comprehensive bioanalytical services. This includes customized solutions based on our LBA and Hybrid LC-MS/MS expertise, ensuring precise evaluation of ADC quantitation to assess efficacy, stability, and safety across preclinical and clinical development stages.

Antibody Drug Conjugate Technologies
KCAS Bio offers a range of advanced technologies to support ADC development including:
Bioanalytical Expertise:
Advanced platforms for quantifying total antibody and intact ADCs, evaluating payload release and quantifying drug-to-antibody ratios (DAR).
Mass Spectrometry:
LC-MS/MS methods for accurate and precise quantitation of payloads, total antibody and ADCs.
Ligand-Binding Assays:
Customized immunoassays to assess immunogenicity and quantitation of total antibody and ADCs.
Regulatory-Ready Solutions:
Compliance with GLP/GCP for submission-ready data packages.
ADC technologies include:
LC-MS
Hybrid LC-MS/MS
LBA (ELISA, MSD)
KCAS Bio conducts a variety of specialized tests to support the development and evaluation of Antibody–Drug Conjugates (ADCs). These include:
Payload Analysis
Quantitation and characterization of the small molecule cytotoxic payload, including its release profile.
Intact ADC Analysis
Assessment of the stability and integrity of the ADC in biological matrices.
Pharmacokinetics (PK)
Delivery of data for modeling the pharmacokinetics of the ADC in test subjects. Non-compartmental PK analysis to determine the ADC’s distribution, metabolism, and elimination profiles.
Immunogenicity Testing
Detection of anti-drug antibodies (ADAs) to assess immune response risks.
Stability Testing
Evaluation of ADC stability under various storage and physiological conditions.
Drug-to-Antibody Ratio (DAR) Analysis
Precise measurement of the average number of payload molecules conjugated to each antibody.
ADCs and Hybrid LC-MS/MS Technology
Recently, we have seen the role of hybrid LC-MS/MS in the analysis of antibody-drug conjugates increase. Antibody drug conjugates tend to consist of a toxic small molecule payload attached via a linker to an antibody. The application of these drugs had mainly been in oncology where their mode of action is for the antibody to attach at the desired target site with subsequent release of the small molecule to combat tumor cells.
Having a therapeutic entity with multiple distinct components means that we need to have analytical methodologies for various components. Traditionally, LC-MS/MS has mainly been applied to the quantitation of the small molecule payload or the linker moiety. Ligand-binding assays had been successful for quantitation of the intact antibody drug conjugate or the antibody portion.
More recently, we have seen a trend towards all analyses being done by LC-MS/MS. Hybrid LC-MS/MS can easily be used to monitor both the total antibody as well as the ADC conjugate. Depending on the type of conjugation and linker used (site-specific vs Cys or Lys conjugation and cleavable vs non-cleavable linker) and what reagents are available, Hybrid LC-MS/MS offers several strategies or advantages to monitor the various constituent parts of the ADC.
Bioanalysis is a continually evolving field. KCAS Bio is focused on applying our LC-MS expertise to be at the forefront of the industry to help address our customers’ analytical challenges. Hybrid LC-MS/MS is proving to be an increasingly valuable tool for delivering answers about complex analytes such as antibody drug conjugates to enhance the drug development process.
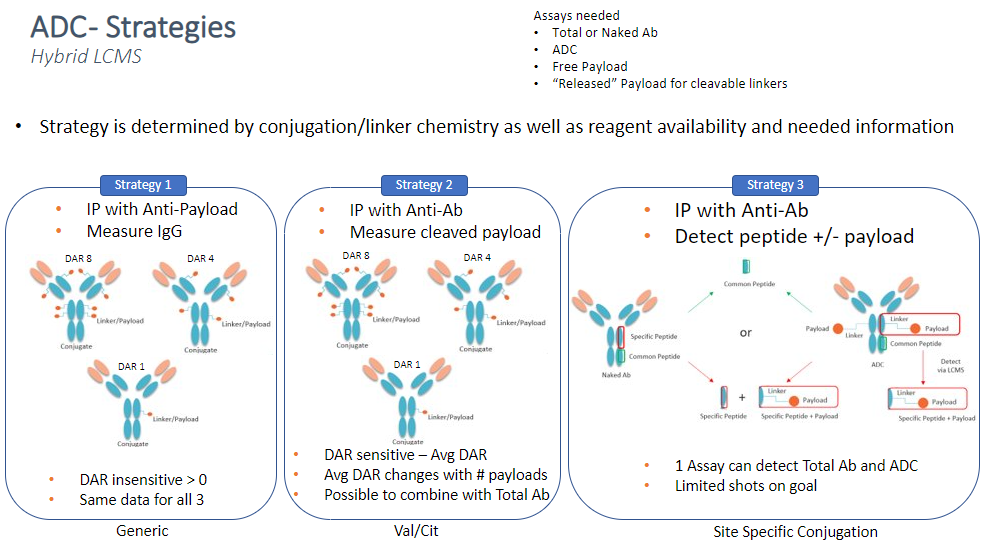
Strategy 1
Capture with anti-payload antibody then digest and detect surrogate peptides from IgG or Specific peptides as needed. This approach is good when there is a good antibody against the payload. This method will also give a positive response for ADC with any DAR greater than zero, therefore this assay would be considered DAR insensitive. This approach can be used as a generic method for any ADC with similar payloads useful during early development to help identify stable ADC’s or lead candidates.
Strategy 2
Capture with anti-ID or Target specific to the antibody, then cleave off the linker/payload and measure the “released” payload as a measure of the ADC concentration. This approach only works for cleavable linkers. The strategy would be considered DAR sensitive and give some additional information about average DAR.
Strategy 3
Capture with anti-ID or target specific to antibody (similar to Strategy 2) and then digest and detect surrogate peptide with or without linker/payload. This approach can also be multiplexed to measure a common peptide (potentially in the FC reagent) as a measurement of total antibody. This strategy is most useful when the conjugation is site specific with limited attachment sites as well as when the linker payload does not have a large molecule weight. This approach can yield 1 assay for both ADC and total Ab.
Let KCAS Bio accelerate your ADC program
Our team of experts provides comprehensive bioanalytical and development solutions tailored to meet your unique needs. Specializing in Antibody-Drug Conjugates (ADCs) and other complex therapeutics, we offer a wide range of specialized services to support every stage of your development journey, delivering precise and reliable results.
Our people
Related services
Resources & Insights
 Blogs
Blogs
Anyone who has been following KCAS for any amount of time has likely heard us discuss the advantages of Hybrid LC-MS/MS for the bioanalysis of large molecules. As pioneers in the technology for many years, KCAS has become a strong proponent of working with our customers to demonstrate the quality…
 webinars
webinars
As the pharmaceutical industry continues to evolve, so does the technology behind drug development. One of the most groundbreaking advancements in bioanalysis is Hybrid LC-MS/MS technology, which is rapidly becoming the new standard for analyzing Antibody Drug Conjugates (ADCs). KCAS Bio is proud to be at the forefront of this…
 Podcasts
Podcasts
In episode 84 of “The Weekly Bioanalysis”, our special guest, Bryan Parmentier, joins Dom and John to discuss his background and current role at KCAS Bio, offering insights into the field of small molecule method development. He delves into the typical challenges encountered when working with LC-MS analytes, and how…

Agile, responsive and easy to work with
We prepare and adapt our services based on a deep understanding of your drug development ambitions and wider business objectives.