How does immunogenicity testing work?
By systematically narrowing from broad detection to functional relevance, our immunogenicity testing workflow at KCAS Bio provides the reliable data needed to support safety, efficacy, and regulatory expectations.
Start a Conversation

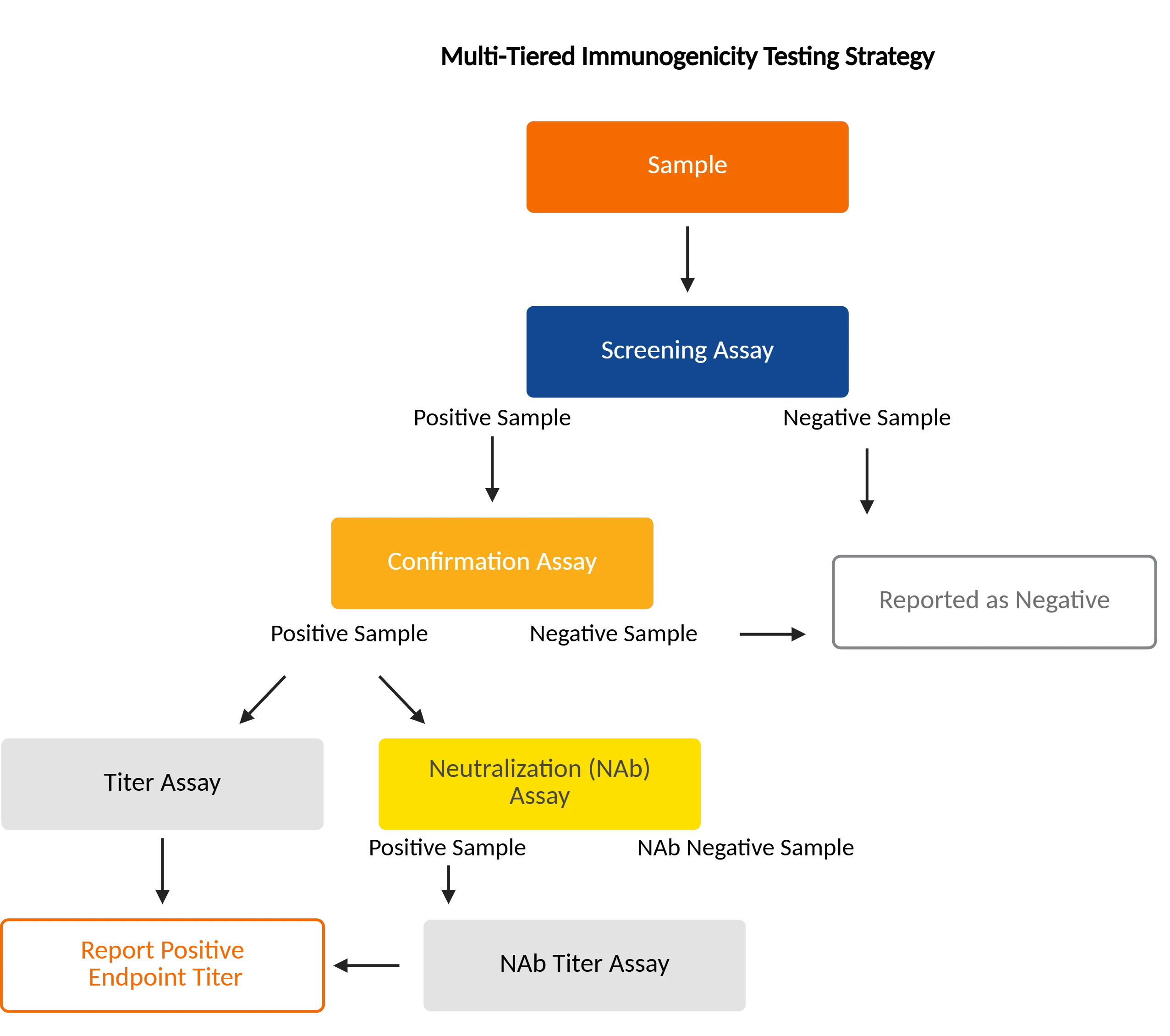
Immunogenicity Process
The immunogenicity testing process follows a structured progression, moving from initial ADA detection to full functional and safety characterization:
-
Screening AssayAll samples are first tested to identify potential ADA-positive results.
-
Negative ReportingSamples that do not show ADA activity are reported as negative.
-
Confirmation AssaySamples that screen positive move to confirmation to verify true ADA binding.
-
Negative ReportingSamples that do not show ADA activity in the confirmatory assay are reported as negative.
-
Titer AssaySamples that confirm positive move into a titering assay. When needed, samples are evaluated for neutralizing potential to see whether ADAs interfere with the drug’s function.
-
Neutralizing Antibody (NAb) AssayNAb-positive samples are further titrated to assess the strength of neutralization.
-
Tiered WorkflowThe process ensures accurate detection, confirmation, and characterization of the immune response to the therapeutic.

Tell us how we can help with your project
We've earned our reputation for delivering reliable, error-free data. We understand the importance of speed, flexibility, and consistency and only make promises we can keep.
Talk to an expert