Spectral Flow Cytometry on a Global Scale
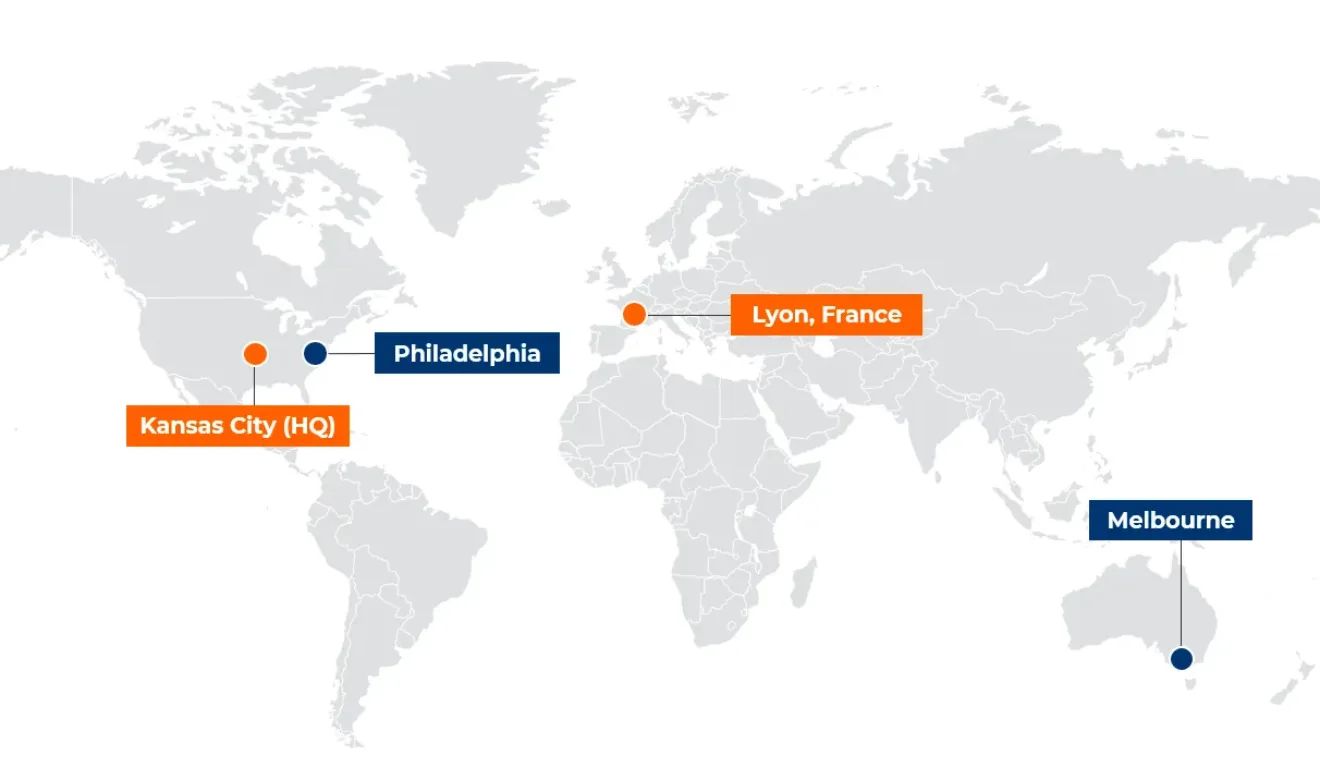
KCAS Bio is the only bioanalytical laboratory with globally harmonized 5-laser Cytek spectral flow cytometers capable of developing, validating, or transferring an assay at any of our US-PHL, Europe, or APAC locations.

Globally harmonized network of spectral flow cytometers
KCAS Bio offers an expanding reach to sponsors who are looking for global support with spectral flow cytometry. Specifically, sponsors now have access to harmonized spectral flow cytometry across the US, Europe, and Asia Pacific. KCAS Bio has invested significantly into all sites, from both an instrumentation and procedural standpoint.
Bringing Crux Biolabs into the alliance enables KCAS Bio to further meet customer demand and accelerate harmonized processes to support advanced flow cytometry capabilities using Cytek Aurora flow cytometers.

The Alliance
- KCAS Bio Spectral Flow Cytometry Instruments at all sites.
- Organizational alignment from the bench to the C-Suite.
- Single contract to govern all sites.
Operations
- Single analytical plan and data transfer process.
- Central project and scientific oversight with direct site access as needed.
Quality
- Consistent GCLP compliance between all sites.
- Central quality review of data and procedures.
Harmonization Process
Multiple levels of comparison to ensure multi-site alignment.
-
Factory AlignmentCytek Aurora instruments reduce the need to adjust voltages and instrument settings between projects. Comparison to predicate instruments across all channels for alignment.
-
Harmonized Set UpPerformance qualification including 40-color and 15-color panels to ensure complete parameter mapping. In person training visits between sites ensure nothing is lost in translation.
-
Quality OversightSOP, worksheet, reagent, and control material alignment through central planning group. Centralized analysis for robust and consistent quality control.
The KCAS Bio Advantage
Complete Provider
- Collaborative environment to facilitate custom assay development
- Fast turn-around time supporting aggressive timelines
- A wide breadth of capabilities, from early discovery to clinical study support
- Multidisciplinary team with expertise in:
- Sample preparation
- Custom Assay Development
- Advanced data analysis
Experience
- Proven expertise in providing sophisticated yet elegant solutions to support functional assay studies.
Since 2018:
- Supported Pre-clinical programs through the development of more than 30 different assays
- Supported >100 clinical-stage programs with immunophenotyping and cell sorting
Service and Quality
- Milestone-based process for efficient & robust study execution
- Quality management team with controlled environment
- GLP compliant capability
Keeping you informed:
- Dedicated Project Manager
- recurring meetings with project scientist
- summary minutes after every meeting
KCAS Bio has Chosen Crux Biolabs as it's APAC Partner
KCAS Bio has embarked on significant investments in spectral flow cytometry, including the placement of harmonized Cytek Aurora cytometers at Crux Biolabs. As a full-service bioanalytical CRO in Melbourne, Australia, Crux Biolabs is well-positioned to support sponsors and clients through the clinical trial journey – from pre-clinical through full clinical development. Australia is well known for its advantageous Phase 1 clinical trial capabilities and this alliance will allow clients to develop flow cytometry assays for use throughout the clinical trial lifecycle.

Experience to Accelerate Your Project
Number of Spectral Experienced Staff
Combined Years of Spectral Experience
Number of Projects to Date
Maximum Colors per Panel
CytoChex, NaHep, PBMCs, Cultured Cells, Urine
Human, NHP, Murine

Tell us how we can help with your project
We've earned our reputation for delivering reliable, error-free data. We understand the importance of speed, flexibility, and consistency and only make promises we can keep.