
Hybrid LC-MS/MS
Hybrid LC-MS/MS is an analytical technique which combines an enrichment step with the selectivity and sensitivity provided by LC-MS/MS.

A track record in Hybrid LC-MS/MS
KCAS Bio is an industry leading expert in Hybrid LC-MS/MS - also known as immunoaffinity or affinity capture LC-MS - with experts in the field who have pioneered this technology for the last 25 years. Hybrid LC-MS is an excellent complimentary approach to ligand binding assays (LBAs). KCAS Bio is a strong advocate of assessing the relative merits of available technologies to answer a project’s needs and using that decision to drive project support.

Principles of Hybrid LC-MS
Hybrid LC–MS only requires one antibody, whereas a typical LBA technique will require two. Following affinity capture, it is common to include a digestion step to produce surrogate peptides, which are representative of the protein of interest and are used for quantitation. The overall process takes advantage of the combined selectivity of the extraction and the flexibility of tandem mass spectrometry to differentiate the analyte from other potential interferents. Our highly experienced scientists use appropriate state-of-the-art technologies as needed to best support all aspects of large molecule bioanalysis which include multidimensional chromatography (high flow to micro flow), bead based enrichments using automated platforms such as KingFisher Flex or other liquid handling instrumentation, anti-protein and online custom anti-peptide antibody enrichments platforms (columns/beads) coupled with high sensitivity MS instrumentation (SCIEX 7500s and SCIEX 6500s).
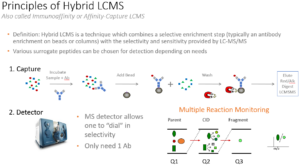
An in-depth look at Hybrid LC-MS
Why Hybrid LC-MS/MS
Hybrid LC-MS/MS approaches are increasingly being utilized to support complex biotherapeutic modalities and can be a great alternative to ligand binding assays. Combining the principles of ligand binding for target analyte enrichment with LC-MS/MS provides an alternative platform for quantitation of large molecules and in some cases becomes the preferred approach. LC-MS/MS can provide significant advantages to quantitate large molecules and at KCAS Bio, we have seen a number of examples where LC-MS/MS provided a clear path forward when an LBA approach met significant hurdles. KCAS Bio has supported many modalities using Hybrid LC-MS/MS including ADCs, mAbs, Fusion proteins, Bi- or Tri-specifics, antibody fragments (Fab, ScFc, etc) and more recently Antibody Oligo Conjugates (AOCs). Hybrid LC-MS/MS is becoming the technology of choice for many ADCs or AOCs.
Hybrid vs Traditional LC-MS/MS
The main difference in Hybrid vs Traditional LC-MS quantitation is the lack of an immunoprecipitation (IP) or enrichment step with critical reagent needed in traditional LCMS. Traditional LC-MS/MS typically involves direct protein digestion (from matrix) followed by a post digestion clean up like solid phase extraction (SPE) or other sample prep cleanup step at the peptide level. This approach is advantageous when there are not good reagents available for LBA or Hybrid strategies. The addition of an enzymatic digestion to produce a range of characteristic peptides provides surrogates that are more compatible with the mass range of many quantitative MS instruments.
How can Hybrid LC-MS/MS benefit you?
Hybrid LC-MS/MS can benefit you and your project’s needs because it can represent the best or only choice in many scenarios. Having many choices helps to ensure we pick the best option for our clients. Hybrid LC-MS/MS can be a game changer when traditional ligand binding assays have failed for a variety of reasons including interference, drug tolerance etc. Leveraging the need for only 1 reagent offers many creative solutions not available to LBA.
When is Hybrid LC-MS/MS the best choice for Large Molecule Bioanalysis?
• Lack of availability of reagents does not prevent development of an LC-MS/MS assay
• Feasible to develop LC-MS/MS assay with one or no good reagents
• Method feasibility/development can start with short lead times
• Use selectivity from extraction, chromatography and MS/MS to avoid interferences
• Ready translation between species with some sequence changes. Less concern of effect on Ab binding
• Ability to differentiate between endogenous (replacement therapy) or human drug in pre-clinical background
• Ability to work around and detect simple sequence changes (e.g. – 1 amino acid, phosphorylation)
• Flexibility in analysis – not tied to a single molecular entity – dial in selectivity by picking different surrogate peptides
• Ability to provide multiple data assessments at any time - Multiplexing
• Same protein but different parts of the molecule or different proteins
Our highly experienced scientists use appropriate state-of-the-art technologies to best support all aspects of large molecule bioanalysis which include various ligand binding platforms or Hybrid LC-MS approaches, as well as Flow cytometry and molecular technologies.
Why is KCAS Bio the best choice for your LC-MS/MS project?
At KCAS Bio, we truly partner with our clients to understand the specific project drivers and what is needed to support their drug development requirements. We try to evaluate each project by starting with the assessment of feasibility in LC-MS/MS, LBA, or both. We can do this in series, or in parallel, depending on the urgency of the project. We like to be able to evaluate these complimentary technologies so we can “pivot” with the client if needed based on the quality of data that each approach provides. At KCAS Bio, both these service lines are co-located in our Olathe, KS facility, which simplifies pivoting between techniques as opposed to working with an organization that only has 1 option or various platforms across multiple locations.
Common questions from our customers
What is Hybrid LC-MS or Immunoaffinity LC-MS/MS?
How do you know when to use Hybrid LC-MS vs. LBA?
Can Immunoaffinity LC-MS/MS, Hybrid LC-MS, help develop an assay when traditional techniques have failed? (SPE, PPT, LBA)
Can Immunoaffinity LC-MS/MS, Hybrid LCMS, help understand more information about the molecule that doesn’t show up in traditional LBA methods?
What type of sensitivities can this approach provide?
View our resources section to learn more about Hybrid LC-MS. Here you will find blogs, webinars, and other information from our veteran scientific experts.
Tell us how we can help with your project
We've earned our reputation for delivering reliable, error-free data. We understand the importance of speed, flexibility, and consistency and only make promises we can keep.
Considerations for incorporating Hybrid LC-MS/MS
1. Generic mAb PK by Hybrid LC-MS/MS – A good example is how we’ve changed our approach to help early discovery work. Similar to using ligand binding assays for generic mAb detection, we have developed a generic approach for quantitating human mAbs in non-clinical species. This means we have very limited development time as we move between different projects enabling rapid data delivery. We can test a new construct and run a batch of samples many times within 1-2 weeks from start to finish. Generally, we focus on Fc peptides but we can add additional more specific regions of the antibody as well and get even more useful data at the same time.

2. The additional flexibility in not being tied to a particular binding site for hybrid LC-MS/MS can also be used to give valuable structural information. During a recent LBA study we identified instability in a biological analyte that interfered with the LBA assay quantitation. Pivoting to hybrid LC-MS/MS, we identified unique peptides at the N-terminus, C-terminus and the center of the molecule. Monitoring those peptides over time we were able to identify the specific region of the molecule that was associated with instability. Furthermore, we were able to confirm that the activity of the therapeutic was not affected by instability in that region.
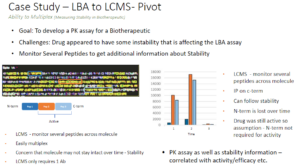
ADCs and Hybrid Technology
Recently, we have seen the role of hybrid LC-MS/MS in the analysis of antibody-drug conjugates increase. Antibody drug conjugates tend to consist of a toxic small molecule payload attached via a linker to an antibody. The application of these drugs had mainly been in oncology where their mode of action is for the antibody to attach at the desired target site with subsequent release of the small molecule to combat tumor cells.
Having a therapeutic entity with multiple distinct components means that we need to have analytical methodologies for various components. Traditionally, LC-MS/MS has mainly been applied to the quantitation of the small molecule payload or the linker moiety. Ligand-binding assays had been successful for quantitation of the intact antibody drug conjugate or the antibody portion.
More recently, we have seen a trend towards all analyses being done by LC-MS/MS. Hybrid LC-MS/MS can easily be used to monitor both the total antibody as well as the ADC conjugate. Depending on the type of conjugation and linker used (site-specific vs Cys or Lys conjugation and cleavable vs non-cleavable linker) and what reagents are available, Hybrid LC-MS/MS offers several strategies or advantages to monitor the various constituent parts of the ADC.
Bioanalysis is a continually evolving field. KCAS is focused on applying our LC-MS expertise to be at the forefront of the industry to help address our customers’ analytical challenges. Hybrid LC-MS/MS is proving to be an increasingly valuable tool for delivering answers about complex analytes such as antibody drug conjugates to enhance the drug development process.
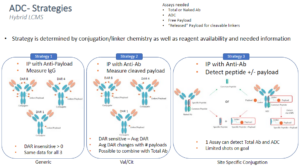
Strategy 1
Capture with anti-payload antibody then digest and detect surrogate peptides from IgG or Specific peptides as needed. This approach is good when there is a good antibody against the payload. This method will also give a positive response for ADC with any DAR greater than zero, therefore this assay would be considered DAR insensitive. This approach can be used as a generic method for any ADC with similar payloads useful during early development to help identify stable ADC’s or lead candidates.
Strategy 2
Capture with anti-ID or Target specific to the antibody, then cleave off the linker/payload and measure the “released” payload as a measure of the ADC concentration. This approach only works for cleavable linkers. The strategy would be considered DAR sensitive and give some additional information about average DAR.
Strategy 3
Capture with anti-ID or target specific to antibody (similar to Strategy 2) and then digest and detect surrogate peptide with or without linker/payload. This approach can also be multiplexed to measure a common peptide (potentially in the FC reagent) as a measurement of total antibody. This strategy is most useful when the conjugation is site specific with limited attachment sites as well as when the linker payload does not have a large molecule weight. This approach can yield 1 assay for both ADC and total Ab.
Our people
Related services

Bringing technical innovation to time-critical research
KCAS Bio partners with leading technology providers so our scientists and clients have access to gold-standard, cutting-edge technologies.