Antibody drug conjugates (ADCs) are important targeted therapies in oncology. These are complex moieties consisting of a cell-targeting antibody combined with an active payload through a chemical linker.
Key Components to Monitor in PK Studies
Two significant components to monitor for PK studies are the (1) intact antibody drug conjugate and (2) the free payload. Payloads are usually cytotoxic so premature separation from the ADC could cause systemic issues for patients. Free payload assays typically require increased sensitivity to detect trace levels of the active moiety.
Challenges in Free Payload Assay Validation
Historically, validations have treated free payload assays as a stand-alone method with development focused on providing sensitivity, accuracy and precision for the assay at hand. After successfully completing multiple ADC projects, it has become apparent that characterizing the free drug payload’s relationship to the ADC is essential. It is also important to understand that a payload assay for one ADC is not always necessarily transferrable to another ADC with the same active drug. The chemistry of the delivery system and the release mechanism for the active ingredient can often be different.
Addressing Concentration Inequalities in ADCs
Fundamentally, the intact ADC is often present at significantly higher concentrations than the payload in study samples. An infinitesimal change in ADC concentration due to analyte degradation can result in a significant effect on downstream payload concentrations. Any assay should be developed with this inequality in mind. Experience shows that the majority of payloads are stable throughout typical sample handling procedures but the same cannot be said for the antibody drug conjugates that contain them. As an example, we have developed assays for multiple ADCs with monomethyl auristatin E (MMAE) as the payload. In our experience, MMAE is stable in biological fluids at room temperature but when samples are processed under those conditions, degradation of the ADC often results in MMAE concentrations well above the LLOQ, when no MMAE should be detectable.
Mitigating ADC Degradation During Sample Processing
Processing samples on ice and using chilled solvents during processing is essential to maintain control of ADC degradation. It’s vital that we assess samples containing the ADC for a payload assay during typical sample processing so we can get a good handle on any potential limits of stability. Instability of the ADC can have a substantial impact on the sample handling instructions given to clinical and non-clinical facilities that will take samples from study subjects.
Based on our extensive experience with ADCs, our payload method development now goes beyond a typical small molecule assay as summarized below.
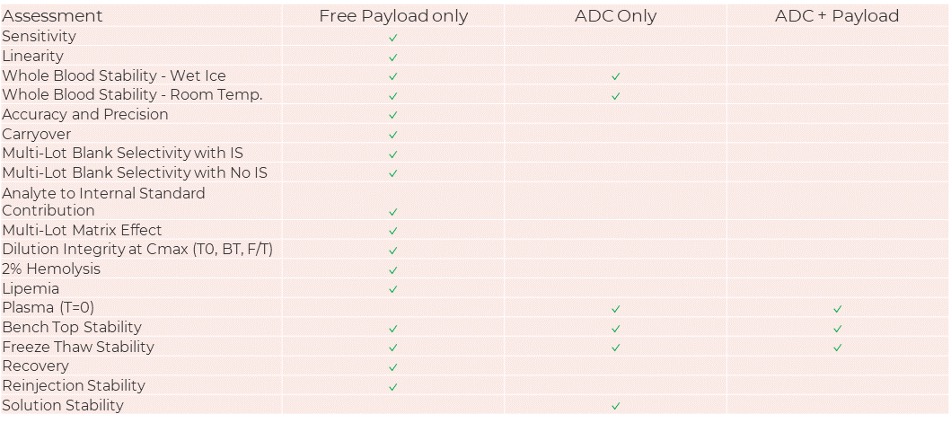
Our validations also assess the effect of sample processing of the ADC on payload concentrations. We are actively discussing whether validation activities should incorporate additional experiments beyond these, as part of our evolving approach to payload methods.
Testing samples spiked with ADC only or with ADC and payload in stability experiments gives a good handle on the likely effect on measurement of the payload. In practical terms, failure at the clinic to follow precise sample handling instructions, based on our observations during development and validation, can result in failed ISRs during clinical trials.
Considering Practical Constraints in Clinical Settings
An additional consideration for method development is that the patient facilities may not have the range of equipment that we have access to. Flexibility in finding alternative approaches such as use of Armor beads for flash freezing instead of dry ice or storage at -20 °C rather than ultra-low temperatures may also need to be assessed.
Leading the Way in ADC Bioanalysis
KCAS Bio has built a wealth of knowledge about the bioanalysis of ADCs through the activities of our ligand-binding assay (LBA) and LC-MS/MS teams. Additionally, our involvement and leadership in panels and conversations taking place around the globe put KCAS Bio at the forefront of the developing science of ADCs.