What are receptor occupancy assays?
Receptor occupancy (RO) assays as designed to quantify and characterize the binding profile of therapeutic drugs to their targets on the cell surface. Typically, these drugs are antibody-based therapeutics that target specific receptors in order to modulate downstream signaling.
With the rise in precision medicine, biotherapeutic drug development now accounts for more than 70% of IND filings, and this is only set to increase as pharmaceutical and biotech companies invest heavily in protein, antibody and nucleic acid-based therapeutics [1]. The premise of using an antibody to modify the action of a receptor is nothing new, but our increased knowledge in antibody engineering and large-scale production has now opened the possibility for widespread applications in medicine [2].
Although antibody-based receptor binding drugs are by nature ‘targeted’ therapies, in order to ensure these drugs are safe and effective, it is still necessary to understand their target specificity, pharmacokinetics (PK) and pharmacodynamics (PD). Typically used with these is RO that essentially measures how a biotherapeutic binds to its specific target. Collectively these measurements may help us understand or confirm the mechanism of action (MOA) and identify any potential safety concerns.
Many biologic therapeutics currently on the market or in development work by binding to a specific cell surface receptor. These therapeutics are typically stand-alone monoclonal antibodies, or antibodies conjugated with a second therapeutic component [3]. One of the key analyses for determining the efficacy and therapeutic dose of these types of biologic therapeutics is the so-called receptor occupancy (RO) assay. Flow cytometry-based RO assays are a powerful and rapid method that can be customized to your cell type and therapeutic molecule of interest.
There are various types of RO assay designs that can measure different parameters of the drug-receptor interaction [4]. The selection of the RO assay format is driven largely by the mechanism of action as well as the availability of reagents.
The 5 most used types of receptor occupancy assay design:
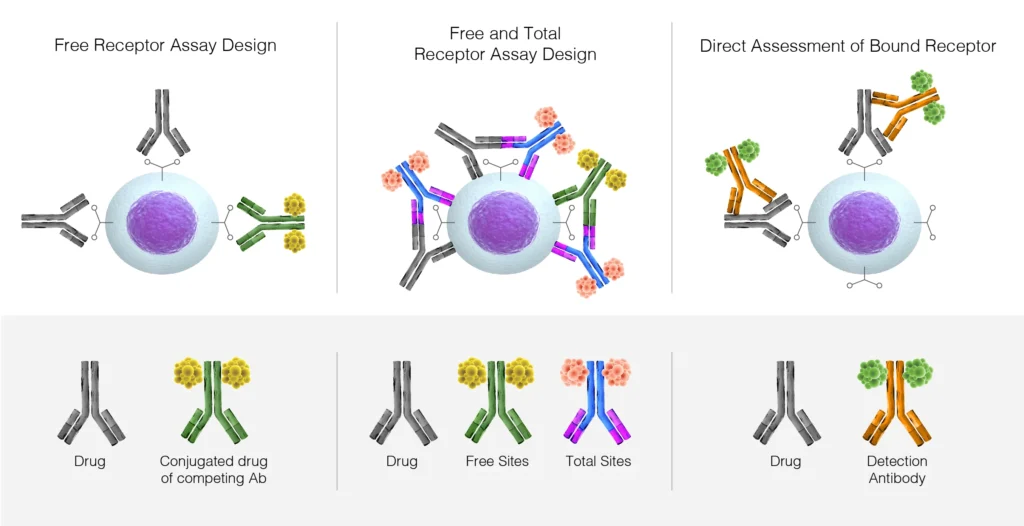
1. Free receptor assay:
This assay measures unbound or unoccupied receptors on a cell by using an unlabeled antibody and a competing therapeutic antibody or drug-conjugated antibody. The binding of the unlabeled anti-receptor antibody is easily measured by using a labeled secondary antibody, allowing for the calculation of unoccupied receptors. This is a critical measurement when assessing the cell specificity and therapeutic dose of an antibody and is typically employed for antagonistic biotherapeutic drugs that block ligand binding to the receptor and the downstream signaling events.
2. Total receptor assay:
This assay measures the binding of a therapeutic antibody to its receptor site, and couples this with the binding of an alternate antibody that binds to a different site on the same receptor. This combination enables the measurement of total receptor expression on cells as well as providing insight into the level of receptor internalization resulting from therapeutic antibody binding. These types of measurement can be useful in clinical trials where the receptor level or cell numbers can markedly change (up or down) over time. A total receptor assay is most commonly employed for biotherapeutic drugs with a mechanism of action that involves up or down-regulation of the receptor, or alternatively, cell ablation by Antibody-Dependent Cellular Cytotoxicity (ADCC) or mobilization of receptor-expressing cells.
3. Bound receptor assay:
This utilizes an anti-therapeutic antibody detection agent (typically a secondary antibody) to directly measure the therapeutic antibodies bound to the receptor of interest and provides critical insight into therapeutic dose measurements and therapeutic antibody stability. In cases of low expression of the target receptor, or expression of the receptor on rarer cell types, then this type of assay can help increase the dynamic signal range through the use of a bright fluorophore such as Brilliant Violet™ on the secondary detection antibody.
4. Functional Receptor Occupancy Assay:
Flow cytometry is an effective way to examine the resulting biological attributes of the therapeutic antibody binding to the target receptor; these include cell proliferation and cytokine signatures, that may have important implications in drug safety and efficacy.
5. Receptor Modulation Assay:
This measures the effect of the binding of the biotherapeutic on the function of the target receptor, such as shedding events or inhibiting (or potentially increasing) the internalization of the receptor particularly over time or as dosing increases.
RO Assays can provide valuable insights at all stages of the drug development process:
Pre-clinical Target verification and MOA. Determining RO through development is critical since many of these biologics have long half-lives, and so understanding their binding characteristics can have an impact on lead compound selection.
Phase I Typically this class of drug is very potent and improper dosing protocols can potentially result in severe side effects or even be lethal. The identification of Minimal Anticipated Biological Effect (MABEL) model starting doses or Pharmacologically Active Doses (PAD) may require RO assessments in conjunction with PD and PK in order to appropriately guide dosing protocols.
Phase II efficacy of dosing and administration protocols to help predict the levels of RO and whether the receptor is modulated up or down on cells that are engaged by the biotherapeutic.
Phase III population PK for long term safety and efficacy studies.
So, what is the foundation of a good RO Assay?
Well at KCAS Bio, we routinely develop fit-for-purpose RO assays based on the specific needs of your study, sample and drug type.
First and foremost, we account for the intrinsic day to day variability of flow cytometry testing. All of our instrumentation is standardized across sights to ensure the performance of the assay in our different labs. We always measure the frequency of target populations, and the quantitative fluorescence intensity of labelled reagents (MESF) must be assessed and compensated for on a daily basis.
Whenever designing an RO panel, the selection of optimal antibody clones and fluorochrome combinations based on the assay requirements is critical. For long term studies, consistent lot use, or lot-to-lot comparisons can be useful, and all instrumentation is appropriately maintained and Quality Controls for instrument performance are conducted daily. In addition, the experiment is designed to employ appropriate background controls such as isotype and fluorescence minus one (FMO) [5].
The stability of the samples over time is a critical limitation of any clinical trial [6], but for RO assays, this stability can limit the design criteria of the assay and should be assessed thoroughly (multiple timepoints, samples, and analysts) before commencing the study. The impact of different processing methods should also be examined [7].
The selection of anticoagulants can have a marked effect on the cell stability, receptor expression level of even the binding of the drug to the target receptor. The use of commercially available stabilizing reagents (such as Transfix™ or Cyto-chex™) or the preparation of PBMCs may have an impact on the dynamic equilibrium of the free and biotherapeutic-bound receptors and should be characterized before selection [7].
Receptor shedding or Internalization is also a potential issue for clinical trial studies measuring RO. At KCAS Bio we have implemented many approaches such as shipping the samples to the lab on ice packs, pre-treating the cells with sodium azide or protease inhibitor cocktails as well as performing the RO assay at 4˚C, to minimize these effects.
Interpretation of RO data
Typically, RO is calculated and reported in terms of % saturation using the ratio of free receptor versus the total number of receptors measured. However, other outputs include % bound receptor expression, reported by utilizing secondary detection antibody that binds to the receptor-bound therapeutic. Various mathematical models have been described in the literature, but essentially RO can be expressed using the calculation below:
Antibody- Receptor Complex [AR]
RO= ——————————————- = ——
Total Receptor [Rtotal]
KCAS Bio’s Fit for purpose approach to RO validation may include the measurement of downstream modulation such as phosphoflow and T-cell phenotypic responses, in order to better understand the effect of the biotherapeutic on target receptor activation.
Learn More
Looking for a complex or customized RO Assay solution? Contact us to discuss your assay needs and we can work together to design a complete solution. Our services include complete RO assay design, optimization, validation, and clinical trial support, along with a wide range of complementary flow cytometry solutions.
2. Curr Pharm Des. 2006;12(14):1785-95. Screening the receptorome yields validated molecular targets for drug discovery. Roth BL1, Kroeze WK https://www.sciencedirect.com/science/article/abs/pii/S0163725804000488?via%3Dihub
3. Adv. Therap. 2019, 2: 1800091. Advances in Receptor-Mediated, Tumor-Targeted Drug delivery. D.E. Large, J.S. Soucy, J. Herbert, D. T. Auguste. https://onlinelibrary.wiley.com/doi/epdf/10.1002/adtp.201800091
4. Cytometry Part B (Clinical Cytometry) 90B: 110-116 (2016) Role of Receptor Occupancy Assays by Flow Cytometry in Drug Development. J.J. Stewart et.al. https://onlinelibrary.wiley.com/doi/epdf/10.1002/cyto.b.21355
5. Cytometry Part B (Clinical Cytometry) 90B: 117-127 (2016) Receptor Occupancy Assessment by Flow Cytometry as a Pharmaceutical Biomarker in Biopharmaceutical Development. M. Liang et.al. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5042057/pdf/CYTO-90-117.pdf
6. American Association of Pharmaceutical Scientist 18(2): 290-293 (2016) Sample Management: Recommendations for Best Practices and Harmonization from the Global Bioanalysis Consortium Harmonization Team. M.J. Redrup et. al. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4779093/
7. Cytometry Part B (Clinical Cytometry) 90B: 150-158 (2016) How validated receptor occupancy flow cytometry assays can impact decisions and support drug development. M. Moulard and M-L. Ozoux. https://onlinelibrary.wiley.com/doi/full/10.1002/cyto.b.21320
{{cta(‘e980b250-b062-43b3-8ec1-5b2983425c9d’,’justifycenter’)}}