Flow Cytometry utilizes fluorescently labeled antibodies to detect specific biomarkers on the surface and within cells, and over the past few years, there has been a surge in reagents available for flow cytometry applications. Most of these have been developed using monoclonal antibodies raised in mice and conjugated to a range of fluorophores. However, there are still instances where suitable monoclonal antibody reagents/conjugates are not commercially available, and small-scale conjugations are not practical. In these instances, so-called indirect staining may be employed, where the binding of an unconjugated primary antibody is detected using a secondary anti-IgG antibody conjugate.
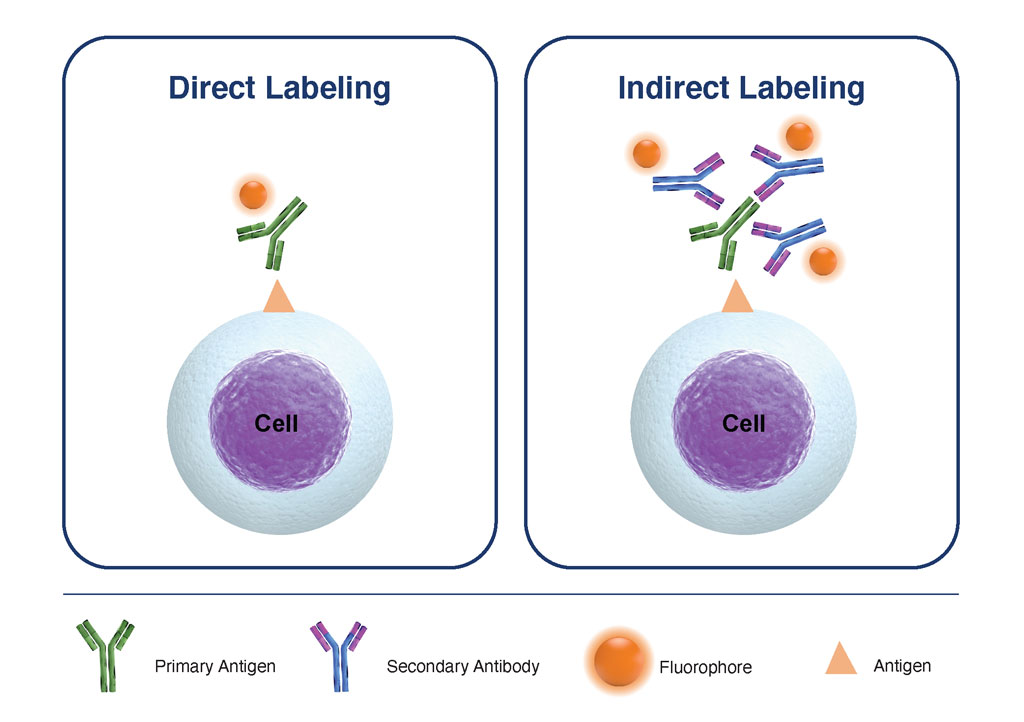
Figure 1. Comparison of Direct Labeling with Primary Fluorophore-Labeled Antibody, versus Indirect Labeling with Unlabeled-Primary Antibody coupled with Fluorophore-Labelled-Secondary Antibody
Experimental Design for Indirect Staining Flow Cytometry Applications
With careful selection of panel reagents and optimization of blocking to prevent Fc-binding to cell surface receptors and washing to minimize non-specific binding, indirect staining can provide excellent signal resolution and specificity, many times comparable with direct staining techniques. There are certain requirements though; for multiparameter staining protocols using a combination of primary conjugates and unlabeled primary antibodies for secondary detection, it is critical that these are from different species, or different classes or isotypes to prevent cross-reactivity of conjugated primary antibodies with secondary antibodies. Table 1 summarizes the core considerations for the selection of secondary antibodies for staining methods including flow cytometry, that when followed, can help ensure optimal staining is achieved.
| Characteristic of Secondary Antibody | Selection Criteria |
|---|---|
| Host- and Target Species | The host species is the animal from which the secondary antibody is generated. The host species should always be different from the primary antibody host and target species. Since most primary antibodies are generated in mice or rabbits, anti-mouse and anti-rabbit IgGs raised in goats are the most widely available types of secondary antibodies. |
| Target of Binding/Reactivity | Ensure the secondary antibody only targets the intended primary antibody-i.e. appropriate species and class or subclass specificity. |
| Purification Process | Secondary antibodies can be purified from serum using a range of processes including affinity ligand binding chromatography (highest level of purity) or using Protein G to enrich the IgG fraction of immunoglobulin (minimum requirement for flow cytometry applications). |
| Cross-Absorption | Cross-absorption involves passing the ligand-affinity purified antibodies over a column of immobilized antibodies or serum proteins from other species- the process helps to reduce species cross-reactivity and minimize background from non-specific secondary antibody binding. |
| Antibody Class and Subclass | Select a secondary antibody that targets the class &/or subclass of the primary antibody in use. This is especially important for the labeling of monoclonal primary antibodies. |
| Whole Antibody versus Antibody Fragment | Applications such as intracellular staining versus cell surface staining may benefit from using smaller secondary antibody fragment- conjugates that provide greater cell penetration and eliminate all Fc-receptor binding. |
| Conjugates | Conjugate selection is a feature of good flow cytometry panel design- large protein-based fluorophores should only be used on conjugates for cell surface labeling. Whereas Alexa Fluor Conjugates and Q-Dot-Conjugates can support robust intracellular staining. Biotinylated primary or secondary antibodies can overcome some of the challenges of poor sensitivity, with the use of avidin or streptavidin secondary probes. |
Table 1. Key factors for the selection of secondary antibodies for flow cytometry staining protocols. Following each of these considerations, will help ensure the selection of the optimal secondary-detection reagent based on the experimental design, target antigens, and cell types.
There are several advantages to the indirect labeling approach, but one of the most valuable for flow cytometry applications is the higher sensitivity that it provides- since more than one labeled secondary antibody binds to each antigen, the signal intensity is amplified. This can be very beneficial when interrogating low-density antigens.
However, there are also some distinct disadvantages to indirect staining such as the complexity in the selection of reagents to minimize false-positive staining effects and higher background if processing methods are not optimized. In addition, secondary antibodies may cross-react with species other than the target, it is therefore critical that both primary and secondary antibodies are titrated, and all incubation, blocking, and washing steps are optimized.
Novel Secondary Staining Antibody-Derived Reagents
In some instances, a novel format of secondary antibodies can be beneficial. Aside from complete IgG molecules, F(ab’)2 fragments, Fab fragments, ScFv, and nanobodies can all be labeled and used for primary antibody detection. In many cases, their use improves the signal-to-noise ratios due to reduced background binding of Fc portions of the antibodies with Fc receptors on cells. A summary of such fragments is provided in Fig. 2. These may be generated through enzymatic cleavage of the complete IgG molecule or engineered in vitro through phage display for example. Their small size, stability, and ease of recombinant expression make these particularly well suited for secondary staining flow cytometry applications, as well as a range of diagnostic and imaging methods.
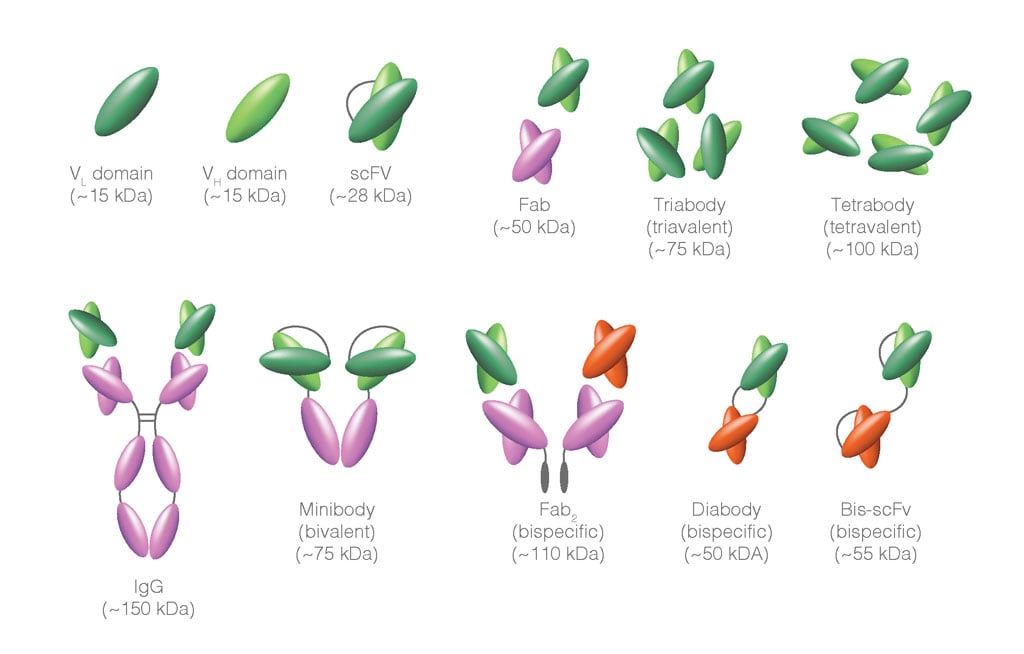
Figure 2. Functional derivatives of IgG molecules. The multi-domain structure of Immunoglobulins can be manipulated to generate recombinant molecules that retain some but not all of the binding functionality of the complete IgG antibody. For example, the variable regions can be preserved and expressed as single domains, or ScFv; these retain antigen-binding activity but without the constant regions, the Fc-binding activity is lost. As with the complete IgG molecule, these proteins can be conjugated to fluorophores for use in secondary detection protocols.
Secondary Detection Signal Amplification
Modification of the primary antibody can also be used to amplify signal strength. One of the most common of these is the use of biotinylated antibodies, coupled with streptavidin or avidin conjugate detection systems. The binding of biotin with streptavidin is highly specific and displays strong affinity, helping to reduce background binding and optimize the signal: noise ratio.
One specialized form of indirect labeling utilizes a 3-layer system to further amplify the labeling signal through the coupling of biotinylated antibodies with the fluorophore-streptavidin conjugate. This can increase signal intensity for the detection of low abundance antigens, with minimal background effects.
Biotinylated antibodies may be used to label bound-primary antibodies in two distinct ways:
- Avidin-biotin complex (ABC) method: large avidin-biotin complexes linked to fluorophores are incubated with biotinylated antibodies. The signal is amplified due to the high fluorophore-to-antibody ratio. This approach is typically only used for cell surface epitope detection.
- Labeled streptavidin-biotin (LSAB) method: the signal is amplified through incubation of the biotinylated antibody with complexes made of streptavidin and fluorophores. Complexes in the LSAB method are smaller than in the ABC method, although are typically not recommended for intracellular staining.
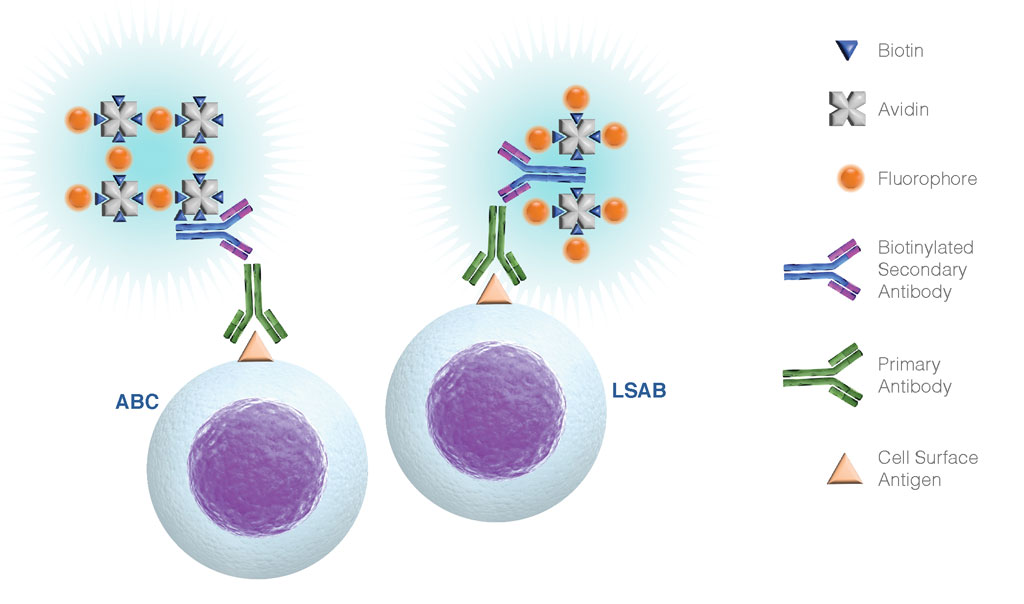
Figure 3. Summary of ABC and LSAB labeling of primary antibodies for cell surface biomarker detection. Both of these techniques add another layer for signal amplification, which can be useful for the detection of poorly expressed surface antigens. The additional layer associates a primary antibody with multiple enzymes or fluorophores, increasing the sensitivity considerably.
Receptor Occupancy Assays Using Indirect Flow Cytometry Staining
Anti-idiotype (anti-IDs) antibodies are antibodies that bind to the variable region of another antibody, typically a biological drug. Since anti-IDs are generally highly specific for the variable region of the target antibody, they are widely used in therapeutic drug development including immunogenicity studies and pharmacokinetic/pharmacodynamics (PK/PD) evaluations. Receptor Occupancy and inhibition of binding assays using anti-idiotypes are now commonly used to support IND filings with the FDA and several distinct flow cytometry methods and analytical algorithms have been developed and optimize for their specific applications (Kohler et. al. 2019). Some examples of these are shown here.
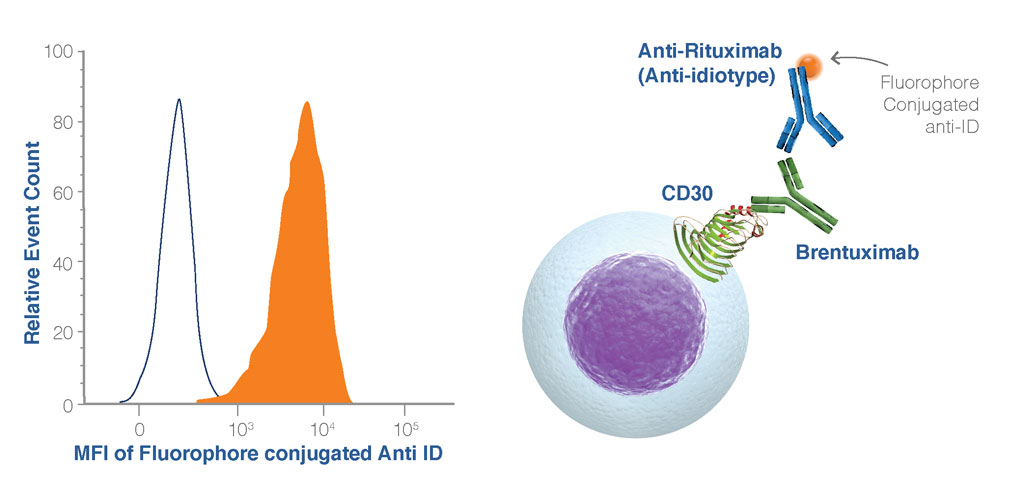
Figure 4. Example of a receptor occupancy readout using an anti-idiotype antibody. In this example, the anti-idiotype antibody binds to the Brentuximab drug antibody bound to its ligand (CD30) on the cell surface (filled histogram). In the absence of the drug antibody, there is no binding of the anti-idiotype antibody to the cell (open histogram).
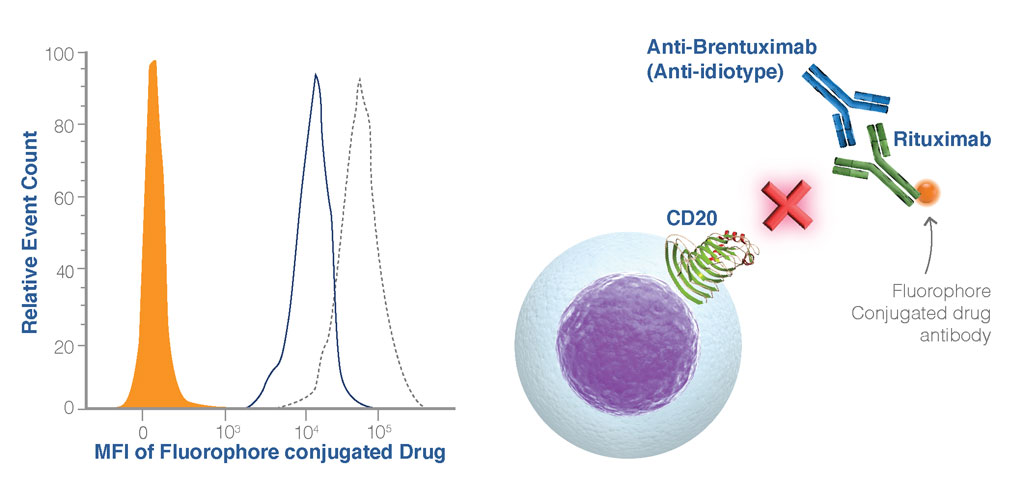
Figure 5. Binding Inhibition Assays. The blocking anti-idiotype antibody binds to the drug antibody preventing binding to its ligand (CD20) on the cell (orange histogram), conversely, in the absence of the blocking anti-idiotype antibody, the drug antibody can bind to the cell (black dotted line). For these types of binding assays, it is important to employ appropriate controls. In this example, an isotype control to the anti-idiotype antibody demonstrates that binding Is specifically blocked by the anti-idiotype antibody (blue line).
Several forms of anti-idiotypes are described based on their binding characteristics, and each of these can be used to assess the performance of the drug for PK/PD using variable flow cytometry-based assay formats.
- Type 1 anti-ID binds specifically to the paratope region of the drug and in so doing inhibits the interaction of the drug and its target.
- Type 2 anti-ID binds to the variable region of drug but is not paratope specifically and does not inhibit the binding of drug and its target.
- Type 3 anti-ID specifically recognizes the complex formed by the interaction of drug and its target(s).
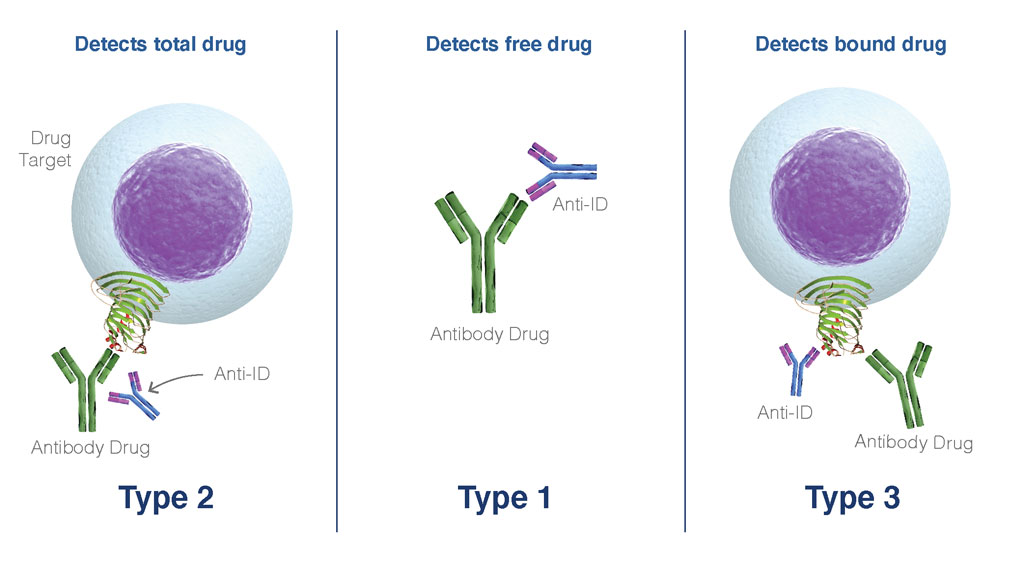
Figure 6. Anti-IDs are useful in the interrogation of the binding affinity and specificity of antibody drugs to their receptor targets. These types of studies can be foundational in assessing the safety and efficacy of biological drugs and the development of anti-IDs is now the focus of growing research activity.
Final Thoughts
For many flow cytometry labs, secondary staining is a last resort and is only used when primary conjugates are not available. However, through careful reagent selection, titration, and protocol optimization, secondary detection can provide excellent signal detection of even antigens with very low-level of expression.
Flow Cytometry methods using secondary staining techniques are now widely used in veterinary medicine and animal health studies where access to species-specific antibody conjugates is much more limited than that of human or mouse reagents (Hunka, et. al. 2020). As with most flow cytometry methods, the key is to understand your experimental system and employ controls to monitor the limitations of this system.
For RO assays, the development and use of anti-IDs are becoming more commonplace. Several types of anti-IDs have now been developed that can be used to describe different features of drug-receptor binding using flow cytometry. With so many immunotherapies now in clinical development, flow cytometry is becoming the gold standard in the assessment of the functional and biological effects of antibody immunotherapies, all using core experimental principles of secondary staining.
References
- Srivastava, P. et. al. (1992) Streptavidin-based Quantitative Staining of Intracellular Antigens for Flow Cytometric Analysis. Cytometry 13:711-721.
- Kohler, H., et. al. (2019) The Promise of Anti-Idiotype Revisited. Front. Immunol. 10:808. https://doi.org/10.3389/fimmu.2019.00808
- Hunka, J., et. al. (2020) Approaches to overcome flow cytometry limitations in the analysis of cells from veterinary relevant species. BMC Vet. Res. Vol. 16: 83.
- https://doi:10.1186/s12917-020-02299-2; PMCID: PMC7060616; PMID: 32143631

