Locked Nucleic Acids (LNAs) are RNA analogs that provide more stable Watson-Crick base-pairing. LNA ribonucleotides can be added into RNA and DNA oligonucleotides to increase the affinity of these oligonucleotides for their complementary sequences. In addition, these LNA present oligonucleotides have extraordinary single-nucleotide discrimination as well as enhanced stability, as each LNA base increases the melting temperature (Tm) of these molecules.
In this blog, I will discuss what LNAs are and how they work, before discussing their potential in PCR-based assays:
What are Locked Nucleic Acids (LNAs) and How They Work:
LNAs were first discovered in 1998 by the Wengel and Imanishi Laboratories. These molecules are recognized by a methylene bridge located within the sugar moiety. The methylene bridge connects the 2’ oxygen to the 4’ carbon of the pentose ring which locks this sugar ring and reduces conformational flexibility of ribose and organizes the phosphate backbone of the nucleotide, hence why LNAs are called “locked” (represented in Figure 1).
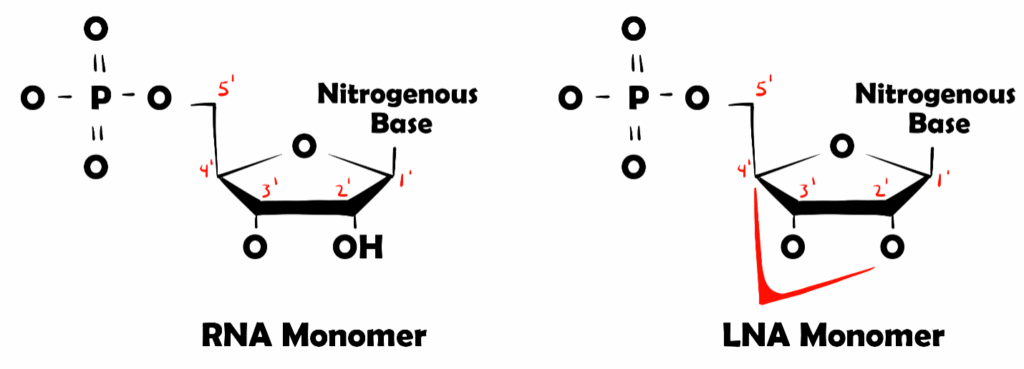
Figure 1. Representation of an RNA Monomer and an LNA Monomer. Methylene bridge of the LNA and carbon primes are highlighted in red.
This conformation restricts and forces the pentose ring of LNA to increase affinity for complementary sequences. Besides increasing affinity, LNAs have low toxicity in animals, increased metabolic stability, good mismatch discrimination, and increased melting temperature for DNA/RNA sequences containing LNAs (Ballantyne et al., 2008).
Incorporating LNAs into Primers and Probes to Develop Sensitive and Specific PCR Assays:
Depending on the obstacles within your PCR assay, LNA monomers have three main features that can greatly improve sensitivity and specificity when developing sequences for PCR Assay primers and probes. These three features are binding affinity, sequence specificity, and thermal stability. Stronger affinity can be useful in matrices with low signal-to-noise ratios such as formalin fixed, paraffin embedded tissues or targeting genes in regions with high GC content. The sequence specificity of LNA substitutions can be used when working on challenging sequences such as detecting single nucleotide polymorphisms (SNPs) or characterizing miRNA expression profiles (Jensen et al., 2011). This specificity can also help when discriminating between large but very similar sequences such as detecting two endogenous genes from the same gene family or discriminating codon-optimized sequences from their endogenous counterparts. LNA present oligonucleotides deliver one of the largest known increases in thermal stability for any edited DNA duplex (Levin et al., 2006). This can be incredibly helpful to increase sensitivity in detecting low target concentrations.
Speaking about thermal stability, one of the greatest difficulties in miRNA expression profiling platforms is overcoming the uncertain Tm of the original miRNA due to wide variation of the GC content from small size oligonucleotides. Introducing LNAs into primers and probes targeting miRNA sequences will increase the Tm of LNA-miRNA duplexes to enhance their robustness without regard for the GC content. This change in Tm is considerably useful when discriminating against similar miRNA sequences as some miRNA families can vary by a single nucleotide. LNA present primers and probes have enhanced detection of less abundant miRNAs than traditional DNA only primers and probes. This is helpful for quantifying miRNA in small volumes of biofluids including human serum and plasma (Jensen et al., 2011).
LNAs can be important tools in PCR-based assays to help increase sensitivity and specificity to discriminate similar sequences such as gene families, orthologs, or paralogs to quantify short sequences such as siRNAs, provide greater mismatch discrimination, as well as just overcoming difficult samples/matrices. However, incorporating LNAs into PCR primers and probes is a complicated task as not only does the addition of LNA substitutions alter the thermodynamics of the PCR chemistry but so does the LNA placement within a sequence alter these thermodynamics. Specifically, the location of LNA substitutions within a primer sequence (near the end versus centrally located) can have a wide margin of differences between Tm values (Levin et al., 2006). While optimum design of primers and probes with LNA substitutions adds a layer of complexity compared to designing traditional primers and probes, this complexity has been made easier over the years thanks to technology and software.
Reach out to KCAS Bio!
LNAs have been demonstrated to be useful in multiple ways for amplification of difficult targets. At KCAS Bio, we are fully capable of designing and optimizing primers or probes containing LNAs for your difficult targets. If you are interested in bringing LNAs to your bioanalysis needs, please reach out to KCAS Bio today!
References:
Ballantyne, K. N., van Oorschot, R. A., & Mitchell, R. J. (2008). “Locked nucleic acids in PCR primers increase sensitivity and performance.” Genomics, 91(3), 301–305. https://doi.org/10.1016/j.ygeno.2007.10.016
Jensen et al. (2011) Evaluation of two commercial global miRNA expression profiling platforms for detection of less abundant miRNAs. BMC Genomics. 12:435. doi: 10.1186/1471-2164-12-435.
Levin, J. D., Fiala, D., Samala, M. F., Kahn, J. D., & Peterson, R. J. (2006). Position-dependent effects of locked nucleic acid (LNA) on DNA sequencing and PCR primers. Nucleic acids research, 34(20), e142-e142.

