Cell therapy clinical trials present unique challenges when it comes to monitoring both pharmacodynamic (PD) and persistence endpoints. Unlike traditional small molecules or biologics, cell therapies involve living products that interact dynamically with the patient’s immune system. Understanding how these cells persist, expand, and function in vivo is critical for assessing efficacy and safety.
One of the most powerful tools for this purpose is flow cytometry, a technology that enables high-resolution, single-cell analysis of complex biological systems. In this blog, we’ll explore how flow cytometry can be applied to PD and persistence analysis in cell therapy trials, using a recent KCAS Bio project as an example, and why high-dimensional flow cytometry offers significant advantages over smaller panels for this purpose.
Why Flow Cytometry for PD and Persistence Analysis in Cell Therapy?
Flow cytometry allows researchers to:
- Track persistence and expansion of therapeutic cells: By labeling or identifying unique markers on infused cells, scientists can quantify their presence in peripheral blood or tissues over time.
- Assess functional status (PD): Beyond counting cells, flow cytometry can measure activation markers, cytokine production, exhaustion profiles, and proliferation indicators—providing insight into whether the therapy is performing as intended.
For cell therapies like those developed by KCAS Clients, which focus on immune effector cells, these measurements are essential. The infused cells must not only survive but also maintain their cytotoxic potential against tumor targets while avoiding excessive immune activation.
KCAS Flow Cytometry Experience with Cell Therapies: A Case Study
Many KCAS Bio clients are pioneering cell therapies for autoimmune therapy or cancer treatment. One example of this is a therapy designed to be “off-the-shelf,” meaning it can be manufactured at scale and delivered without patient-specific customization.
In clinical trials for such products, flow cytometry is used to:
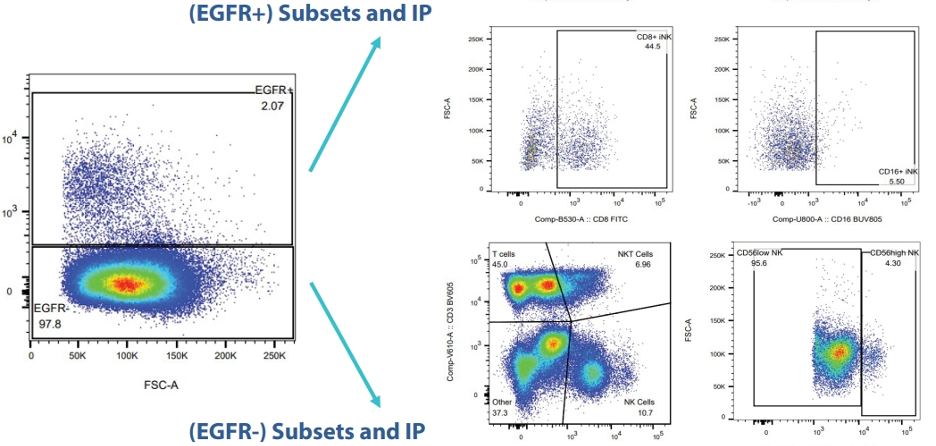
- Confirm cell therapy persistence: Detecting the engineered cells that were injected into patients using custom-developed flow assays and unique client markers (e.g., CAR constructs or starting material-specific signatures). High sensitivity assays are a must to be able to detect cells at a low concentration, especially based on the expanding use of non-depleting administration approaches.
- Evaluate PD endpoints: Measuring activation markers (CD69, CD25), cytotoxic granule release (Granzyme B, Perforin), and exhaustion markers (PD-1, TIM-3) to ensure the cells remain functional.
Expanding PD Endpoints for Autoimmune Therapies
For autoimmune indications, PD endpoints often include depletion of specific immune subsets that drive disease pathology. For example:
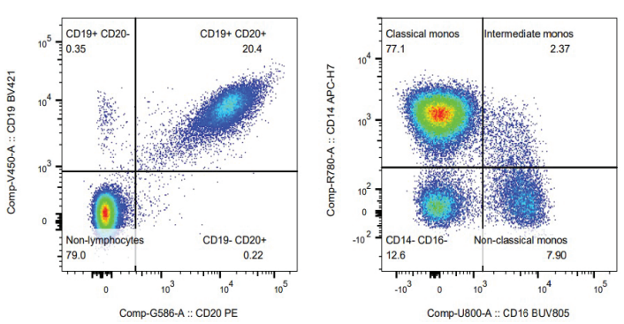
- B-cell depletion: Therapies targeting B cells (e.g., anti-CD19 CAR-T or NK cells) require monitoring CD19+ and CD20+ populations in blood and tissues. Flow cytometry can quantify the degree and duration of depletion, which correlates with clinical response.
- T-cell modulation: In some autoimmune settings, assessing regulatory T cells (Tregs) or pathogenic effector T cells provides insight into immune rebalancing.
- Cytokine signatures: High-dimensional panels can track shifts in inflammatory cytokine-producing subsets, confirming downstream pharmacodynamic effects.
Including these PD endpoints ensures that cell therapies not only persist but also achieve their intended immunomodulatory impact without causing excessive immunosuppression.
The Advantage of High-Dimensional Flow Cytometry
Traditional flow panels with 8–12 colors provide useful targeted information, but cell therapy trials often demand deeper insights. High-dimensional flow cytometry, leveraging 20–40+ parameters per cell, offers several benefits:
- Comprehensive immune profiling: Simultaneously assess therapeutic cells and the patient’s immune response from limited blood volumes (e.g., Tregs, myeloid cells, cytokine-producing subsets).
- Uncover subtle phenotypes: Identify rare populations or transitional states that smaller panels might miss.
- Data-driven biomarker discovery: High-dimensional datasets enable advanced computational analysis (e.g., clustering, trajectory mapping) to correlate cell states with clinical outcomes.
For KCAS clients, this means not only confirming that their cell therapies persist but also understanding how they interact with the tumor microenvironment and the host immune system—critical for optimizing dosing strategies and predicting response.
Conclusion
Flow cytometry is indispensable for monitoring PD and persistence endpoints in cell therapy trials. While smaller panels can address basic questions, high-dimensional flow cytometry unlocks a richer understanding of therapeutic cell behavior and immune dynamics, ultimately accelerating the development of safer and more effective treatments.
As cell therapy advances from oncology into autoimmune indications, integrating high-dimensional flow cytometry with computational analytics will become a cornerstone of translational research and clinical monitoring. KCAS looks forward to remaining the CRO partner of choice for sponsors looking to maximize the data they obtain from each patient visit on their trial.