Immunoaffinity LC-MS/MS or hybrid LC-MS/MS is an approach that has been gaining a lot of traction in the past several years. The technique allows the quantitation of low-level analytes (proteins or peptides in many cases) in biological matrices with exquisite selectivity and sensitivity. Many pharmaceutical companies are trying to expand their expertise in this area and develop sensitive assays for both biotherapeutics as well as biomarkers. KCAS Bio is an expert in this area and has supported many modalities from non-GLP pre-clinical studies to GLP and clinical trials over the last 6 years.
Below are a few questions that we are often asked by customers…
What is Hybrid LC-MS/MS or Immunoaffinity LC-MS/MS?
Hybrid LC-MS/MS is a technique which combines an enrichment step (typically an antibody enrichment on beads or columns) with the selectivity and sensitivity provided by LC-MS/MS. It is called “Hybrid” because it combines steps from traditional ligand binding assays (LBA) and mass spectrometry based assays. Typically, the target/analyte of interest is “captured” either at the protein (or peptide level) using magnetic beads or column-based supports (LBA first step). The analyte (protein) is digested to produce surrogate peptides which are then specifically detected via an LC-MS/MS method (LC-MS/MS 2nd step – detector).
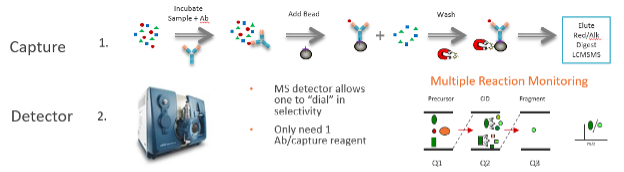
How do you know when to use Hybrid LC-MS/MS vs. LBA?
This is always a good question. The two approaches are very complimentary and, in many cases, either approach will give you the answer. However, there are cases where Hybrid LC-MS/MS might be a better choice. For example, if you don’t have access to great reagents or only one reagent is available then hybrid LC-MS/MS might be a better choice since 1 reagent is all that is needed for the initial capture step. The detection on the back end is via the mass spectrometer. Furthermore, the initial capture step for the hybrid LC-MS/MS approach is only used to help enrich or clean up the sample so the capture reagent does not need to be exclusive for the analyte as you benefit from the selectivity of MS/MS as your ultimate detector. There are also cases where a small sequence change between species may not be detected in an LBA method but the MS can “dial” in the correct sequence so it could offer better translatability. This also helps to support multiplexing various parts of the molecule (in cases of stability concerns) or various analytes (such as 2-3 plex assays for ADCs, bi-specifics or multimodal analytes). Lastly, we have seen examples where there is an interference in the LBA method (could be background interference or drug tolerance issues) which have been circumvented with a LC-MS/MS approach. Ultimately, this question should be discussed at the beginning of a project to help identify the best approach for your specific project. There are many cases where we will do limited method feasibility for a few days using both LBA and LC-MS/MS approaches to generate data to ensure we are choosing the best method prior to moving into method development/qualification/validation for the assay. We firmly believe in taking the path of least resistance and using the more straightforward approach that will provide the answer.
What are the most common modalities or cases where Hybrid LC-MS/MS seems to be the best choice?
We have supported many modalities and projects over recent years and many are what we would call “rescue” projects. These are where either LBA or another CRO LC-MS group was not able to adequately develop the needed assay and Hybrid LC-MS/MS was pursued as the best alternative. In addition to rescue projects, we have found there are a few common themes that seem to be emerging where Hybrid LC-MS/MS might be the best first choice. These modalities include generic mAb assays where we can very rapidly verify how the target drug performs in our standard assay conditions and then can be supported in-vivo PK studies for any preclinical species quickly and cost effectively. ADCs are another popular modality where the analysis has recently been benefiting from Hybrid LC-MS/MS technology. Typically, 3 assays are required for ADC development (ADC, total Ab and free payload). Many times, depending on the type of conjugation and linker chemistry, a single assay can provide both ADC and total Ab by Hybrid LC-MS/MS. We have many case studies illustrating various examples of this approach for ADCs which will be a topic of a future BLOG by KCAS Bio. Another common area for Hybrid LC-MS/MS is in support of protein biomarker projects. These can be complicated by lack of reference standards as well as the need for total/free/bound assays. Many of these needs can be addressed with a flexible approach using different sample preparation strategies in Hybrid LC-MS/MS. Lastly, a newer area where we are leveraging Hybrid LC-MS/MS technologies is for the analysis of other formats of drug conjugates (like Antibody Oligo Conjugates – AOC or ARC) or antibodies coupled to other peptide drugs.
Can Immunoaffinity LC-MS/MS (Hybrid LC-MS/MS) help develop an assay when traditional techniques have failed? (SPE, PPT, LBA)
Absolutely, this is the area where the hybrid LC-MS/MS approach shines. Many times, the background observed using traditional extraction approaches is just too noisy to be able to get a good clean signal for the analyte of interest. The key is removing that background. By using a reagent to enrich the analyte of interest (typically an antibody to the target) you can eliminate the background and enrich for your molecule of interest. This approach can increase your sensitivity by several orders of magnitude. You can also have multiple steps of enrichment. For example, initial enrichment at the protein level with digestion to a surrogate peptide can be followed with additional enrichment at the peptide level. Recoveries at each enrichment step will not be 100% so it is important to evaluate there are sufficient benefits to make sure the additional step is necessary.
Can Immunoaffinity LC-MS/MS (Hybrid LC-MS/MS) help provide more information about the molecule that doesn’t show up in traditional LBA methods?
Yes, since you are using a MS detector as the back end, you know the exact sequence and identity of the peptide you are quantitating. You can easily look at post-translational modifications (assuming they have a different molecule weight/sequence or chromatographic retention time). You can also use this approach to simultaneously monitor several parts of the molecule if you are concerned about a particular area or stability. These assays can very easily be multiplexed for many peptides or even proteins depending on your enrichment strategy.
What type of sensitivities can this approach provide?
I would stack up Hybrid LC-MS/MS as one of the most sensitive approaches for protein quantitation compared to any LBA assay. At the end of the day sensitivity comes down to signal-to-noise. The combination of the capture step with the most sensitive MS can provide impressive selectivities and sensitivities. To get the most sensitive assays, you can also consider chromatography scales (micro- or nano-flow) combined with large injection volumes and multidimensional chromatography. The general approach to get the best sensitivity is to clean the analyte up very selectively (with an antibody), inject as much material as you can, concentrate that on a trap and then introduce it into the most sensitive MS in the narrowest peak (nano- or micro-LC) possible.
KCAS Bio has deep expertise in this area with scientists with over 25-years’ experience. We have expertise in both LBA and Hybrid LC-MS/MS areas within the same lab which enables us to easily pivot to the correct technology as needed. We have the ability to enrich analytes at the protein or peptide level using bead and column-based automation. We can support early Discovery through GLP or regulated preclinical and clinical assays. We love to engage with our clients in delivering the best high-level science to address their most challenging issues.