Data by FlowMetric
All flow cytometry experiments begin with similar basic set up protocols to assure that the equipment is functioning properly and that samples can be measured accurately. Using a flow cytometry gating strategy is an essential step during this set up phase as it assures that the correct cell populations are being measured. Here are factors to consider as you determine how you want to establish your flow cytometry gating strategy for your next experiment.
- What are your cells of interest? Each cell subset is defined by a unique set of cell markers that are found on the surface or within intracellular compartments. When you have determined which cell subsets you want to measure, you will need to define your flow cytometry staining panel to assure that these cells can be measured as separate subpopulations using different fluorescent markers. The staining panel is critical for setting up your flow cytometry gating strategy.
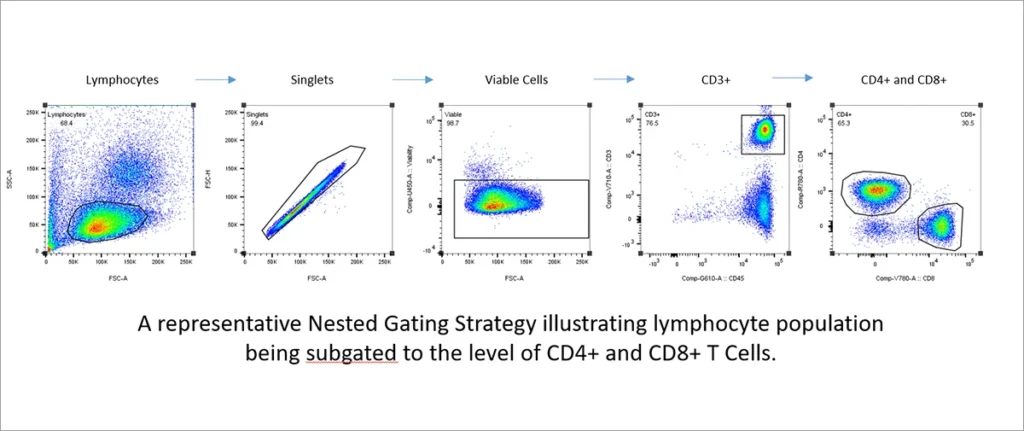
- How will you define your subsets? Most flow cytometry gating strategies use nested gating for which you establish a gate around a broad population, such as viable lymphocytes, and then create a series of gates using the cell subset from each previous gate. This strategy narrows down your populations of interest so when your run your samples, you will be sure to collect a sufficient number of events within the subgate of interest to have a statistically significant sample size.
- Do your gates stay put or shift? Once you have used control samples to establish your gates, these gates should stay fixed as you run your samples so you can assure consistent collection and analysis of data. This is a critical consideration as you evaluate your flow cytometry gating strategy as some samples will appear to shift in where the cell subsets appear relative to gates. Sometimes gating strategies will have to be re-evaluated to make sure all the cells of interest are measured.
- Are you considering all the controls? During flow cytometry set up, other critical controls must be run to make sure the fluorescent dyes are being measured correctly. In addition to compensation controls, fluorescence minus one (FMO) controls are critical to establishing gating boundaries as cells are stained with the entire panel of fluorescent stains except the one for which you are establishing the gate, and this is done for each stain. Isotype controls are also critical for your flow cytometry gating strategy as they can measure non-specific binding of an antibody. The isotype control is different than the antibody used to stain a specific antigen but shares the same isotype.
Together these considerations help you achieve a robust flow cytometry gating strategy and give you confidence in accuracy and reliability of your flow cytometry data.

