Due to its ability to analyze multiple parameters across different cell types within a sample, flow cytometry provides rich and clinically valuable data sets from even small volumes of blood. However, flow cytometry is a challenging platform to master, and requires significant investment in equipment and technical training. So, for many researchers, outsourcing flow cytometry to a Contract Research Organization (CRO) is both cost-effective and the best way to ensure the highest quality of data from their samples.
One of the most routine and yet powerful applications of flow cytometry is immunophenotyping. This involves the analysis of white blood cells by measuring the presence or absence of key biomarkers/antigens, coupled with the characteristic differential light scatter properties of cells. Combined with cell counting beads, immunophenotyping by flow cytometry can be used to enumerate specific cell subsets and has many applications across clinical studies, including the diagnosis of leukemias and lymphomas and the stratification of patient responses within clinical trials. The reliability of immunophenotyping analysis is dependent on several factors, including the quality of the sample and the staining and processing reagents, instrument performance, and the quality of the data analyses. It is therefore always recommended to work with a CRO that has an established Quality Management System underpinned by Standard Operating Procedures across all workflow applications to ensure the quality and reproducibility of the results.
Immunophenotyping for clinical diagnostic applications may require CLIA or CAP accreditation, whereas patient monitoring during clinical trials may be accomplished under the GLP and GcLP guidelines. FlowMetric Life Sciences provides immunophenotyping services under all of these compliance requirements.
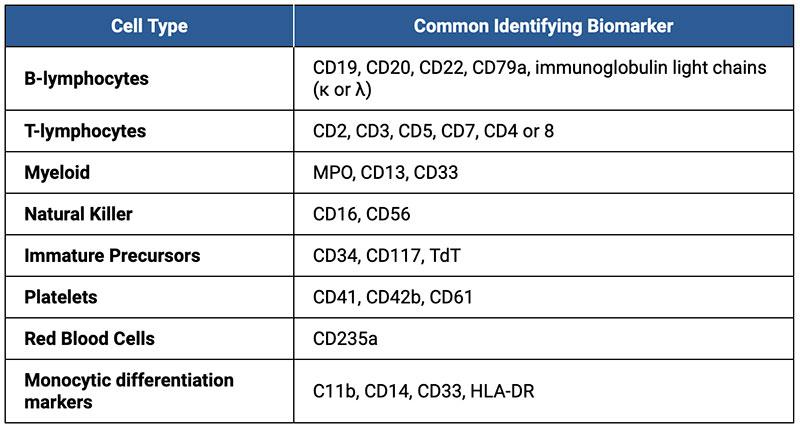
Table 1. Basic markers used for Immunophenotyping applications. Flow cytometry is now the preferred method for lineage assessment, maturational characterization of malignancies, heterogeneity, and quantification of subpopulations (Rothe and Schmitz).
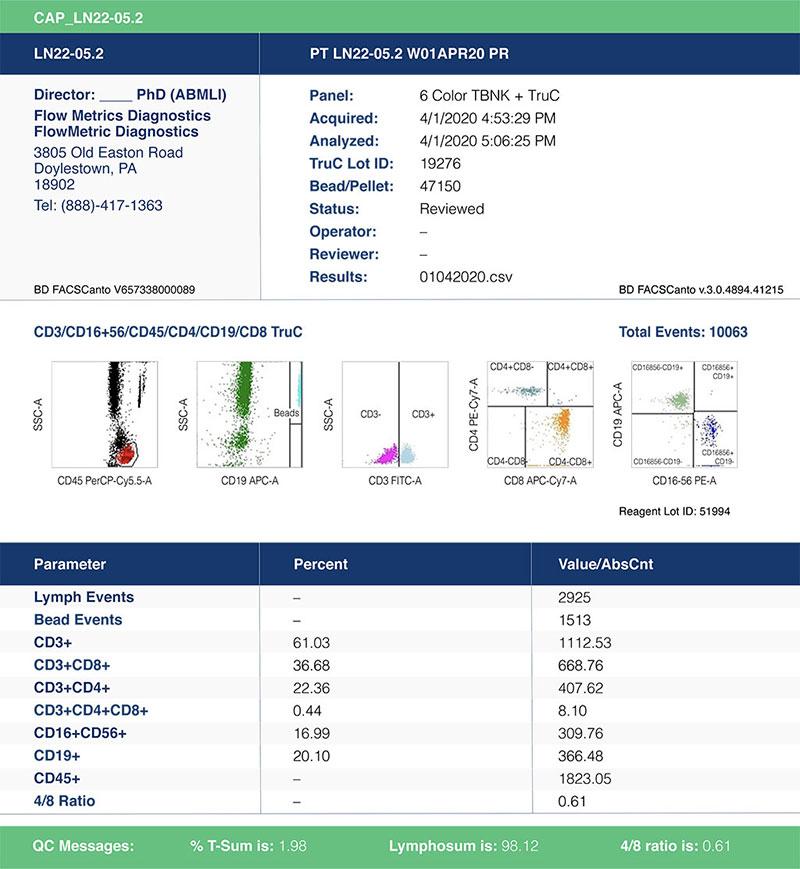
Figure 1. Example of a TBNK report for an immunophenotyping panel performed under CLIA certification, CAP accreditation. The report summarizes both the absolute numbers of T-, B- and NK cells as well as their % within the population.
There is a growing need for flow cytometry assays to support drug development initiatives, largely due to their multiplex nature and subsequent rich data sets. However, the barriers to entry and time needed to develop flow cytometry assays means that outsourcing to a specialized CRO will ultimately save both time and money, all while ensuring the robustness of your data.
The development of novel high-performance fluorophores and cellular probes, coupled with the commercial availability of monoclonal antibodies against an increasing number of antigens means that flow cytometry can provide analytical solutions across a wide range of cellular functions. Working with a CRO who provides the highest quality of flow cytometry services ensures that your research team is tapped into state-of-the-art flow cytometry instrumentation, methodology and analytics to help accelerate your project goals.
If you have any questions for our KCAS team members, please use the form below to contact them.