From initial receipt to final disposition, sample integrity, traceability, and compliance are the foundation of our bioanalytical operations. We’re taking you behind the scenes to follow the journey of a sample through our facility. Here’s a detailed look at each stage in the lifecycle of a study sample at KCAS Bio.
Step 1. Controlled & Secure Receipt at Dedicated Access Points

Study samples arrive through one of two dedicated loading doors, entering directly into a controlled-access warehouse. Immediately upon receipt, packages are directed to our secure accessioning area. Labels or markings differentiate shipments that are addressed to the Principal Investigator (PI) or the TMM (Test Materials Management) group from incoming supplies, ensuring proper routing.
Step 2: Priority Assessment
Samples are segregated by study, and our team of 16 cross-trained, qualified personnel triages incoming shipments to support variable timelines and study requirements, ensuring expedited processing for high-priority programs.
Step 3: Comprehensive Integrity Assessment

Packages are opened under controlled conditions. The following are documented in accordance with SOPs and logged in Watson LIMS (Laboratory Information Management System), creating a permanent, traceable record of your sample’s condition upon arrival.
- Shipping condition verification (temperature monitors, cooling materials remaining, insulation integrity)
- Quantity confirmation
- Container integrity (e.g., signs of breakage or spillage)
Samples are then transferred to a quarantine freezer, where they remain under monitored storage until full data entry and verification are complete.
Step 4. Barcode Association and Positional Logging

Sample barcodes are scanned and linked within Watson LIMS, associating each sample ID with its shipment metadata. Discrepancies (e.g., quantity or labeling mismatches) are flagged, and queries are submitted for immediate resolution.
Upon clearance, samples are placed into positional storage: each sample is assigned a unique location identifier (freezer ID, shelf, rack, box, and cell position) for complete traceability throughout its lifecycle.
Technical Note: Our TMM team manages over 1 million sample transactions annually, including accessioning, racking, retrieval, and final disposition.

Step 5. Sample Pull Request and Transfer to Laboratory
When laboratory staff require samples for analysis, analysts submit a LIMS-based sample transfer request. The TMM team locates the samples, prepares them in appropriate racks, and scans them to the appropriate lab location. Samples are delivered via our secure, internal handoff station.

Step 6. Controlled Laboratory Usage & Cold Chain Compliance During Use
Laboratory personnel have five business days to complete their analysis and return samples to TMM. Samples in use must remain on the analytical bench or be stored in designated interim secure cold storage. All cold storage units and areas are monitored via the REES Centrol Presidio environmental monitoring system, providing real-time alerts for temperature excursions and maintaining GLP compliance. Samples in cold storage are only accessible by authorized personnel.
Step 7. Return to TMM

Upon completion of the analysis, lab staff initiate a sample return request via Watson LIMS. Returned samples are verified by barcode and returned to positional box storage, maintaining our complete chain of custody documentation.
Step 8. Post-Analysis Storage and Final Disposition

Post-analysis, samples remain in long-term storage until study completion. At that point, the Project Manager (PM) consults with the client and PI to determine the final sample disposition. We offer three primary options:
- Sample Return (Dispatch): Samples are returned to the client or location of their choosing using premium courier services (domestic and international) which provide temperature control and tracking throughout transit.
- Secure Disposal: Thermal destruction with our certified partner Stericycle, with full documentation and certificate of destruction provided for your records.
- Extended long-term storage: Continued secure storage with maintained environmental controls and monitoring. Samples are available for retrieval upon request.
Compliance Note: No samples are destroyed or shipped without explicit client and PI authorization.
Additional Services for Sample Management
Periodic Sample Reconciliation
We offer periodic sample inventory reconciliation during the study lifecycle—monthly, quarterly, or at a custom cadence—to verify holdings and maintain alignment with trial records. This mitigates risks, reduces downstream queries, and ensures sample accountability in line with regulatory expectations.
Kitting Services for Improved Efficiency
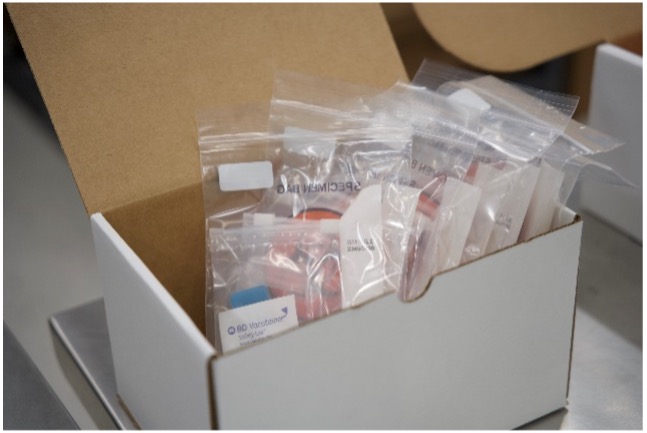
Our in-house custom kitting services can streamline sample submission. We generate pre-populated manifests and sample labels fully integrated with Watson LIMS, improving clinic-to-lab communication and reducing intake discrepancies.
Operational Benefit: Pre-kitting reduces query generation, shortens turnaround time, and minimizes human error during sample receipt.
In conclusion, each sample that arrives at our facility is managed under strict quality systems, from accession to archival or destruction. Our processes ensure regulatory compliance, sample integrity, and clear traceability—supporting your study’s success at every step. Contact us to learn more.

