 June 25
- June 26
June 25
- June 26
KCAS Bio is happy to announce our participation in the 13th edition of the Antibody Industrial Symposium (AIS), taking place in Tours, France. As a premier event in the antibody and therapeutic development sector, AIS offers a unique platform for scientific exchange, collaboration, and innovation. KCAS Bio’s Expertise…
 Posters & Papers
Posters & Papers
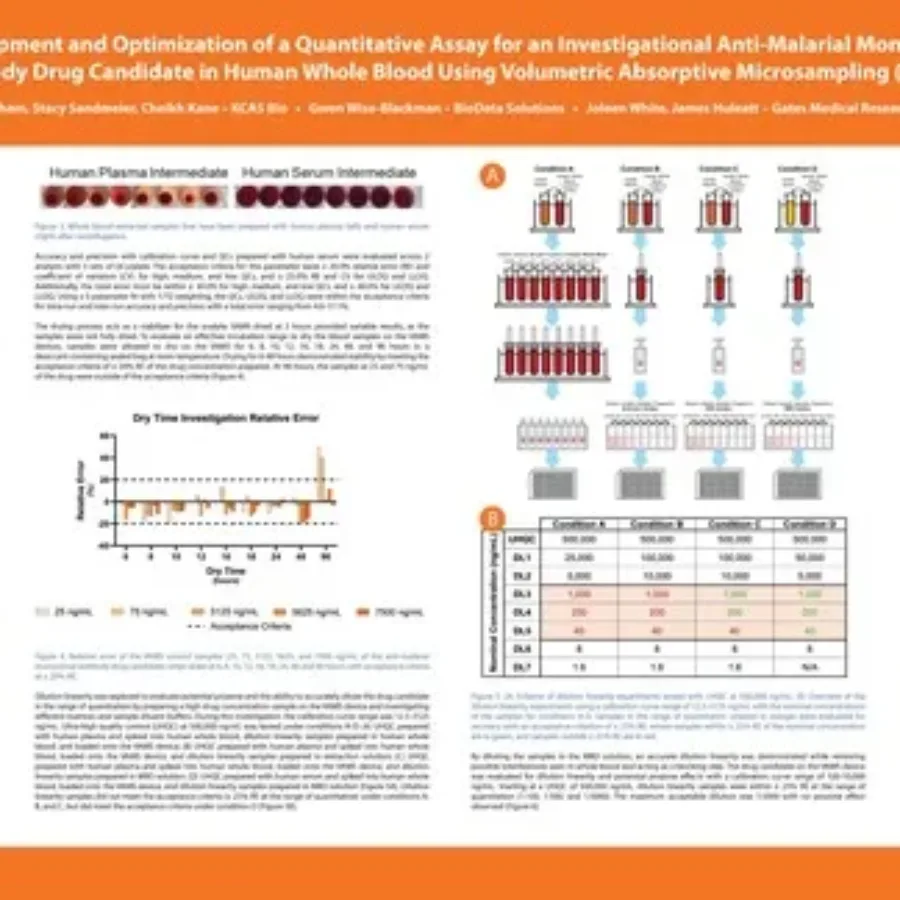
Discover in this poster presented by Jessica Phan at WRIB 2025 on the “Development and Optimization of a Quantitative Assay for an Investigational Anti-Malarial Monoclonal Antibody Drug Candidate in Human Whole Blood Using Volumetric Absorptive Microsampling (VAMS)”. Quantitative Assay for an Investigational Anti-Malarial Monoclonal Antibody Drug Candidate |…
 Blogs
Blogs
Quantitative flow cytometry (QFCM) is a specialized technique that enables precise measurement of the absolute number of specific molecules (e.g., receptors, antigens, or intracellular targets) on individual cells or particles. Understanding flow cytometry is essential, as standard methods typically provide qualitative data, where the relative fluorescence intensity is used to…
 Blogs
Blogs
What Are Neutralizing Antibody (NAb) Assays? Neutralizing antibodies (NAbs) are a subset of anti-drug antibodies (ADAs) that play an important role in evaluating the immunogenicity, safety, and efficacy of a drug product. While most drugs carry a low risk of eliciting NAbs and do not require a validated NAb…
 Webinars
Webinars
In the dynamic field of biotherapeutics, understanding and managing anti-drug antibodies (ADAs) is critical for the success of drug development programs. ADAs can potentially neutralize the efficacy of biotherapeutic drugs, making it essential to have robust testing methodologies in place from the preclinical to clinical stages. KCAS Bio is excited…
 Blogs
Blogs
Georges Köhler and César Milstein: The birth of monoclonal antibodies They discovered the technique for monoclonal antibody production. They won the Nobel Prize in Physiology or Medicine in 1984. They directly led to the development of antibody-based therapies for a vast array of health conditions. Since their initial invention…
 Blogs
Blogs
Ligand binding assays (LBAs) have been our core activity for decades. LBAs are commonly used to measure interactions between two proteins, a ligand and its receptor, a monoclonal antibody (mAb) and its target, or biologics and Anti-Drug Antibodies (ADA). Throughout the development of New…
 Podcasts
Podcasts
Starting with a description of Antibody Drug Conjugates (or ADCs, for short), Dom and John dive into this field of the industry and discuss the ways these services have changed over time and even how they’ve changed recently. They review the role of ADCs in meeting some of…
 Blogs
Blogs
With recent guidance released from the FDA, there are changes for PKs (Pharmacokinetics) and ADCs (Antibody Drug Conjugates) that must be clearly understood before making decisions for your drug product testing. ADCs combine the target specificity of monoclonal antibodies with the…
 Blogs
Blogs
Neurodegenerative diseases affect millions worldwide. Fifty million people are living with Alzheimer’s disease or other dementias. Although Alzheimer’s disease is one of the most recognized, it is just one of many neurological disorders, such as Multiple Sclerosis, Parkinson’s, or Huntington’s disease. These conditions lead to a…
 Blogs
Blogs
Cell and Gene Therapies (CGTs) has an estimated market size value in 2022 of USD 8.22 billion and a revenue forecast in 2030 of USD 24.5 billion. This is a CAGR (compound annual growth rate) of 14.6% from 2022 to 2030. Needless to say, the…
