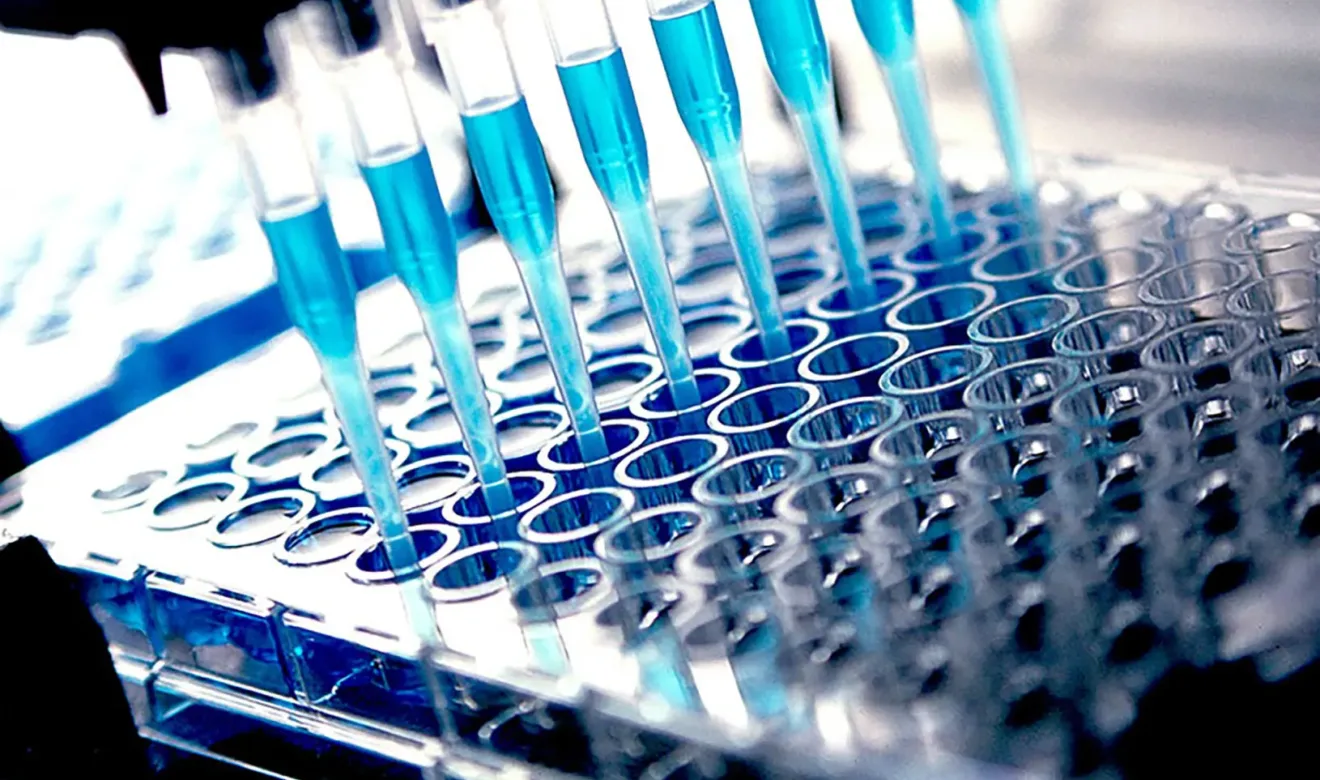
Biomarker Services
KCAS Bio offers a number of analytical platforms to perform single or multiplexed biomarker analysis. We have experience on a variety of matrices (biological fluids & tissues) within a wide range of therapeutic areas.

Bringing the latest technical innovation to biomarker services
KCAS Bio offers considerations about which matrix to use and assay sensitivity. We help clients determine whether a qualified assay can be used to generate sufficient data on a biomarker’s potential usefulness, or if more thorough analytical validation will be required.

Biomarker assay services
The KCAS Bio team is well versed in the full scope of biomarker applications, including:
• In-depth knowledge and experience in method development and kit adaption
• Fit-for-Purpose method qualification/validation
• Single analyte and multiplex analysis
• Measurements in both animal and human matrices
• Nominal or no-cost qualification of most multiplex panels
Biomarker technologies include:
Soluble Biomarkers:
LBA, qPCR, ddPCR, LC-MS/MS, MSD
Cellular Biomarkers:
ELISpot, Flow Cytometry, qPCR, ddPCR, Single-Cell Proteomics
Fit-for-Purpose Biomarker Applications
Our 40 years of experience has prepared us to make your most nuanced project a success. Our approach with fit-for-purpose projects includes:
- Needs-based, fit-for-purpose qualification or validation
- Consultative in determining your needs
- Experience in both method development and kit adaption
- Single-plex and multiplex analysis
- Measurements in both animal and human matrices
- Nominal or no-cost qualification of most multiplex panels
KCAS Bio is biomarker ready
Biomarker assays are an important part of the KCAS Bio full-service assay capabilities. Our dedicated and highly experienced scientists use state-of-the-art technologies for biomarker analysis to support preclinical, clinical and exploratory projects where fast data turn-around may be of essence.
Our people
Related services
Resources & insights
 Blogs
Blogs
A biomarker is a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or biological responses to an exposure or intervention, including therapeutic interventions(1). With the development of innovative and personalized therapies, biomarker tracking is increasingly important to support the clinical development…
 Blogs
Blogs
Tissue and Non-Liquid Matrices (such as cell lines, red blood cells, PBMCs, bone marrow aspirates, and suspension cells) are valuable matrices[] to test for biomarkers (PD) and drug levels (PK). Testing of tissues and…
 Blogs
Blogs
Biomarkers are key drivers in the drug development process. Frequently, developing a panel of biomarkers to test a particular mechanism or hypothesis is critical to the success of a drug. At KCAS, we develop assays for custom panels or individual biomarkers based on client needs and can also assist with…

Agile, responsive and easy to work with
We prepare and adapt our services based on a deep understanding of your drug development ambitions and wider business objectives.